Fig. 7.
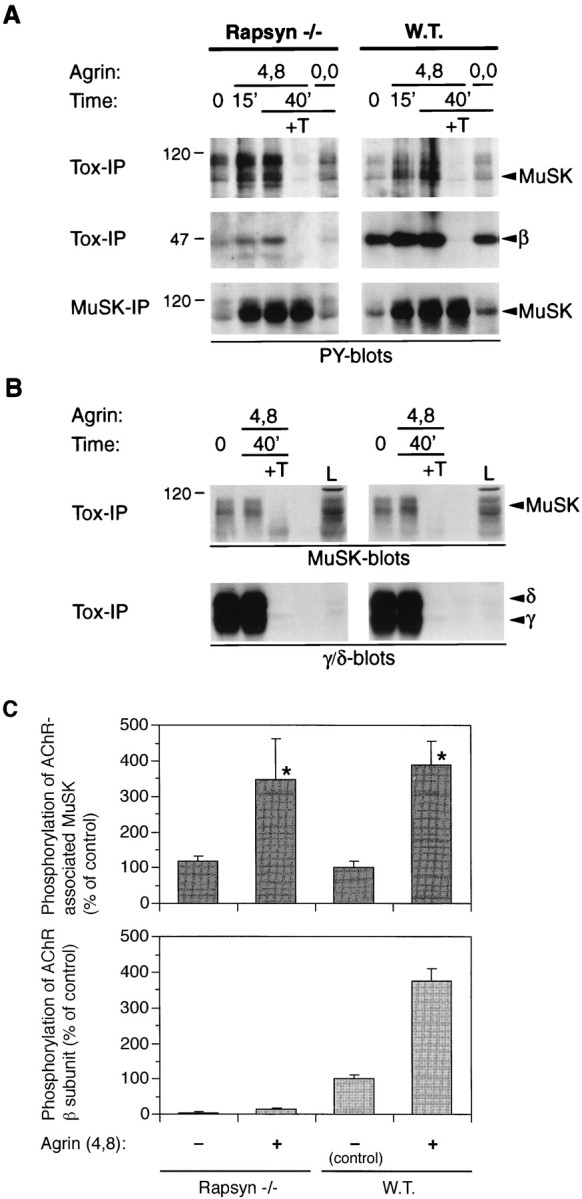
Agrin causes efficient tyrosine phosphorylation of AChR-bound MuSK, but not of the AChR β subunit, in rapsyn −/− myotubes. Rapsyn −/− (11-7) and wild-type myotubes (12-10) were treated with 1 nm neural (4,8) or muscle agrin (0,0) as indicated. A, Lysates were split into two parts and incubated either with α-BT-Sepharose beads (Tox-IP) to isolate AChRs or with MuSK antibodies and protein A-Sepharose beads to precipitate MuSK (MuSK-IP). Samples were analyzed by phosphotyrosine immunoblotting, and proteins were identified by their molecular weight and by stripping and reprobing the blots with the appropriate antisera (data not shown). Agrin causes phosphorylation of total and AChR-bound MuSK in both cell types. Phosphorylation of the AChR β subunit is much stronger in the wild-type cells; a shorter exposure of the blots in the middle section reveals a strong agrin-induced signal in the wild-type cells and no signal at all in the mutant (data not shown). B, Levels of AChR-associated MuSK and AChRs isolated on toxin beads are equal in rapsyn −/− and wild-type cells. AChRs were precipitated from lysates of mutant and wild-type cells, and samples were analyzed by immunoblotting with MuSK or AChR γ/δ-specific antibodies.L, 0.3% of the total lysate was analyzed as a standard.C, Quantitation of tyrosine phosphorylation of AChR-bound MuSK and the AChR β subunit. Immunoblots made from samples treated for 40 min as described in A were quantitated by densitometric scanning of films. Short exposures of films were used that contained nonsaturated signals. The intensities were normalized for the amount of AChR-bound MuSK, as revealed in a toxin precipitation followed by a MuSK blot (B). Values of untreated wild-type cells were set to 100%, and other values were calculated accordingly. Data represent mean ± SD of three experiments. *Differ significantly from control (p < 0.05, by ANOVA followed by Bonferroni’s t test), but not from each other.
