Fig. 8.
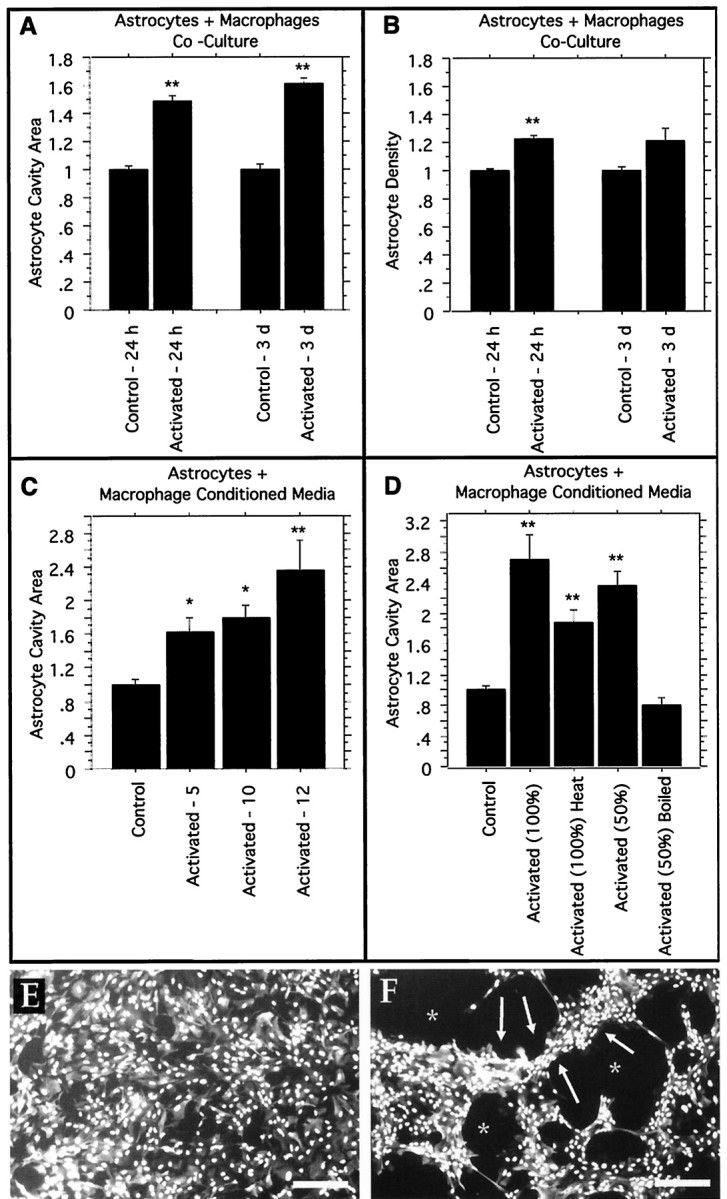
Inflammation leads to astrocyte cavitation in the in vitro astrocyte cystic cavitation model. The area of astrocyte cavity per microscopic field is significantly increased by activated macrophages or activated macrophage– conditioned media. A, B, Astrocytes + macrophages coculture. Astrocyte monolayers were established on poly-l-lysine coverslips, and peritoneal macrophages were added with no activating stimulant [control nonactivated macrophages (Control)] or with zymosan particles [activated macrophages (Activated)]. Control cultures are normalized to a value of 1, and all replicates are combined and expressed relative to their own individual controls. A,Astrocyte cavity area per field of view (areas of the culture that were covered previously by the confluent monolayer of astrocytes and that are subsequently devoid of cells). Significant increases at 24 hr (p < 0.0001) and 3 d (p < 0.0001) in the presence of activated macrophages as compared with nonactivated macrophages are shown.B, The density of astrocytes. A significant increase at 24 hr (p < 0.0001) and a slight increase at 3 d (p = 0.0723) suggest that astrocyte migration may be occurring in the cultures exposed to activated macrophages. C, D, Astrocytes + macrophage-conditioned media. Astrocyte monolayers were established with conditioned media from macrophage cultures, either zymosan-activated macrophages (Activated) or nonactivated control macrophages (Control). Control cultures are normalized to a value of 1. C, Cavity area of astrocyte monolayers grown on laminin in the presence of macrophage-conditioned media for 3 d with increasing numbers of macrophages present during the initial conditioning step (5 × 106, 10 × 106, and 12 × 106macrophages per 10 ml of conditioned media). The significant cavity formation produced by activated macrophage–conditioned media is demonstrated. D, Cavity area of astrocyte monolayers grown on poly-l-lysine in the presence of macrophage-conditioned media for 24 hr with various treatments to the conditioned media. Full-strength conditioned media [Activated (100%)] lead to a significantly larger culture cavity (p < 0.0001), whereas heating that same full-strength media to 60°C for 15 min [Activated (100%) Heat] modestly decreases the cavitation, which is still significantly higher than that in control nonactivated macrophage–conditioned media that have been heated (p < 0.0001). Conditioned media diluted to 50% strength with fresh media [Activated (50%)] retain cavity-inducing activity (p < 0.0001), but boiling the conditioned media for 40 min before 50% dilution with fresh media [Activated (50%) Boiled] abolishes the effects. E, F, Representa-tive photomicrographs of as-trocyte cultures in the in vitrocavitation model stained with GFAP to visualize astrocyte intermediate filaments and with DAPI to visualize cell nuclei demonstrating a typical control (E; nonactivated macrophage–conditioned media from C) and a typical activated (F; activated macrophage–conditioned media from C). Note the even distribution of the astrocyte monolayer in E, whereas F contains areas of increased astrocyte density (arrows) and areas of culture cavity (asterisks). Similar results were seen with the cell coculture experiments reported in A andB. Scale bars, 225 μm. ANOVA with reported significance is relative to the appropriate control nonactivated macrophage preparation or conditioned media, and graphsreport group means ± SEM (*p < 0.005; **p < 0.0001).
