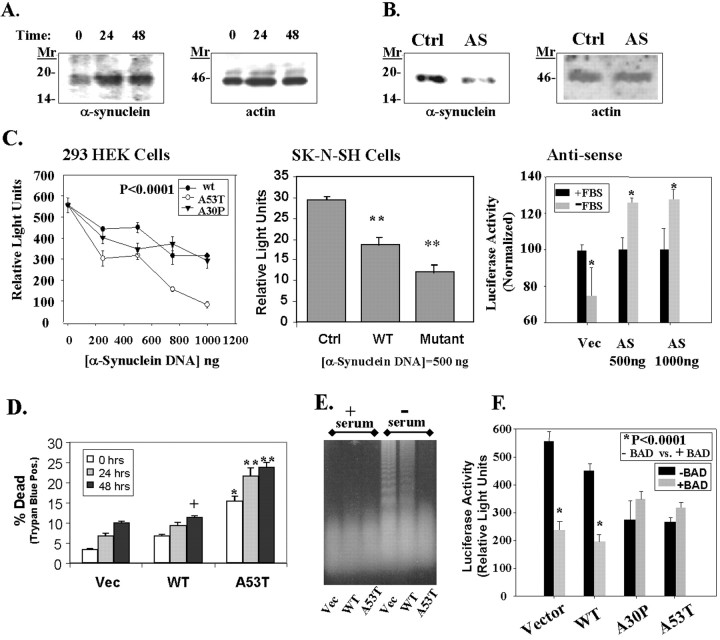
Fig. 6.
Toxicity of α-synuclein. A, α-Synuclein expression increased during incubation of 293 HEK cells in serum-free medium. The left shows an immunoblot of α-synuclein with SC1 antibody, and the right shows the same immunoblot reprobed with anti-actin antibody (Sigma).B, Transfection of 293 HEK cells with an antisense α-synuclein construct (AS; 2 μg) reduced the amount of endogenous α-synuclein expressed in the cells (left). The control lane(Ctrl) shows lysates from cells transfected with an empty pcDNA3 plasmid under the same conditions at the same time. The immunoblot was then reprobed with anti-actin antibody to show that equal amounts of protein were loaded in each lane(right). C, Transient transfection of 293 HEK (left) or SK-N-SH cells (middle) with a pGL3 luciferase plasmid and vector, wild-type, A53T, or A30P α-synuclein constructs induces a dose-dependent decrease in luciferase activity. In contrast, transfection with antisense α-synuclein increased luciferase expression (right). For the antisense experiments in the right, cells were transfected and then serum-deprived for 24 hr, after which luciferase activity was measured. Cells transfected with antisense α-synuclein showed less toxicity than cells transfected with vector. *p < 0.05; **p < 0.01;n = 4 for each point. D, Similar experiments showed a dose-dependent increase in toxicity as shown by trypan blue staining after serum deprivation for 0, 24, or 48 hr. Parallel experiments with a β-galactosidase vector showed a 40% transfection rate in 293 HEK cells. Based on 20% of the cells being positive for trypan blue after transfection, we estimate that transfection of 1 μg of A53T α-synuclein induced ∼50% cell death in 293 HEK cells. +p < 0.05; *p < 0.01; **p < 0.001.E, Effects of α-synuclein on DNA fragmentation. No fragmentation was seen under basal growth conditions in the control cell line (Vec, lane 1), wild-type α-synuclein overexpresser (WT, lane 2), or A53T α-synuclein expresser (A53T, lane 3). After 24 hr incubation in serum-free medium, oligomeric DNA fragmentation was strong in the control cell line (lane 4), moderate in the wild-type α-synuclein cell line (lane 5), and absent in the A53T α-synuclein cell line (lane 6). A slight increase in highly degraded DNA is apparent in lane 6 above the dye front, suggesting increased necrotic DNA. F, Wild-type α-synuclein increases BAD toxicity, but mutant α-synuclein (A53T and A30P) does not increase BAD toxicity. 293 HEK cells were cotransfected with the constitutively active 1 μg of pGL3 luciferase plasmid with or without 100 ng of BAD, and with or without 500 ng of α-synuclein (wild-type, A53T, or A30P). A β-galactosidase vector was used as a ballast to maintain the DNA amount at 2 μg; this vector does not affect luciferase activity. *p < 0.0001; n = 4; comparing samples with or without BAD. The A53T and A30P transfections alone were also significantly different from vector at p < 0.0001;n = 4.