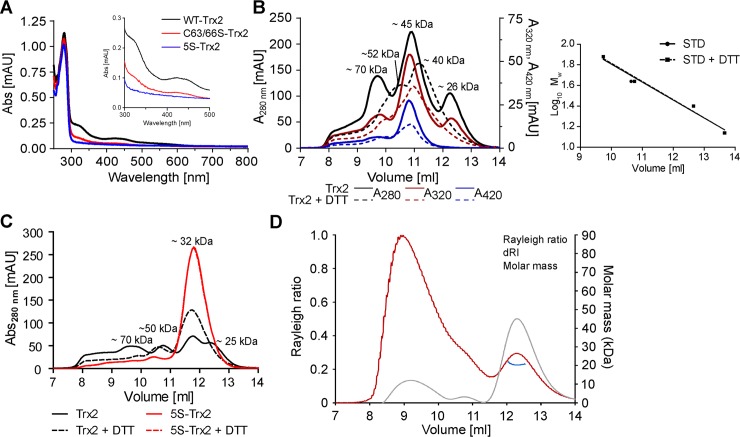
Fig 5. Spectroscopic properties and oligomeric state of recombinant Trx2 species.
(A) The UV-visible spectra of 120 μM Trx2, C63/66S-Trx2 and 5S-Trx2 in 50 mM sodium phosphate, 300 mM sodium chloride, pH 8.0. (B and C) The gel filtrations were performed in 50 mM sodium phosphate, 150 sodium chloride, pH 7.0 on a Superdex 75 10/300 GL column. Trx2 and 5S-Trx2 were incubated for 30 min at 25°C with and without 25 mM DTT, centrifuged for 45 min at 13,000 rpm and 4°C, and the protein concentration in the cleared supernatant was determined. (B) 25 μl of pre-reduced and 50 μl of untreated Trx2 (1.2 mM), respectively, was loaded onto the column which was run at room temperature and a flow rate of 0.3 ml/min in the presence or absence of 1 mM DTT. The elution profile was recorded at 280, 320, and 420 nm. (C) 25 μl of untreated and pre-reduced Trx2 (0.88 mM) and 50 μl of 5S-Trx2 (0.43 mM) pre-incubated ± DTT were processed as outlined above. Protein elution was followed at 280 nm. The apparent molecular masses of the peaks were calculated from standard chromatograms. (D) 100 μl of 80 μM 5S-Trx2 was subjected to size exclusion chromatography with online multi-angle light scattering (SEC-MALS). Relative intensity of Rayleigh ratio (red) and differential refractive index (dRI, grey) from the light scattering detector are shown against the elution volume. The determined mass (blue) is shown for the second peak.