Figure 1. ER-PM Tethering.
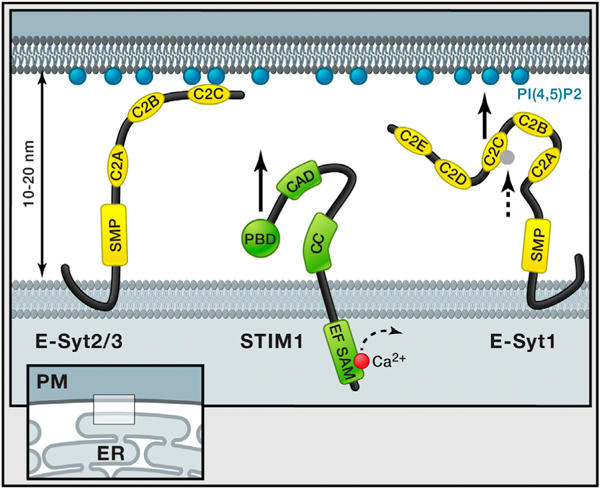
ER-PM junctions are ubiquitous cell compartments In which the two membranes are stably kept at a distance of 10 to 20 nm. Giordano et al. (2013) show that three ER-localized proteins, E-Syts, play an important role in tethering the ER to the plasma membrane. Overexpression of E-Syt2 and E-Syt3 connects large regions of the ER to the plasma membrane mediated by E-Syt C2C domains that bind plasma membrane PI(4,5)P2 independent of Ca2+. In contrast, E-Syt1 is at basal Ca2+ levels not associated with the plasma membrane, but, as the intracellular Ca2+ level increases, the C2C domain will bind Ca2+ (dashed arrow), resulting in binding to PIP2 in the plasma membrane (black arrow) and trapping of E-Syt1 at the ER-PM junction. This is very different from the regulation of STIM1, for which previous studies showed that STIM1 translocation to these same ER-PM junctions is mediated by lowering of lumenal ER Ca2+ levels (illustrated with dashed arrow), which triggers its oligomerization and the exposure of a PI(4,5)P2 binding and an Orai interaction region (black arrow). Giordano et al. (2013) further show that the previously predicted transmembrane region at the N terminus of E-Syts forms a hairpin insertion in the ER membrane, making both N and C termini accessible to the cytosol. All E-Syts have a cytosolic SMP domain, whereas E-Syt1 has five C2 domains compared to three for E-Syt2 and E-Syt3. Domains depicted on STIM1; EF, EF hand; SAM, sterile α motif; CC, coiled-coil; CAD, CRAC activation domain; and PBD, polybasic domain.
