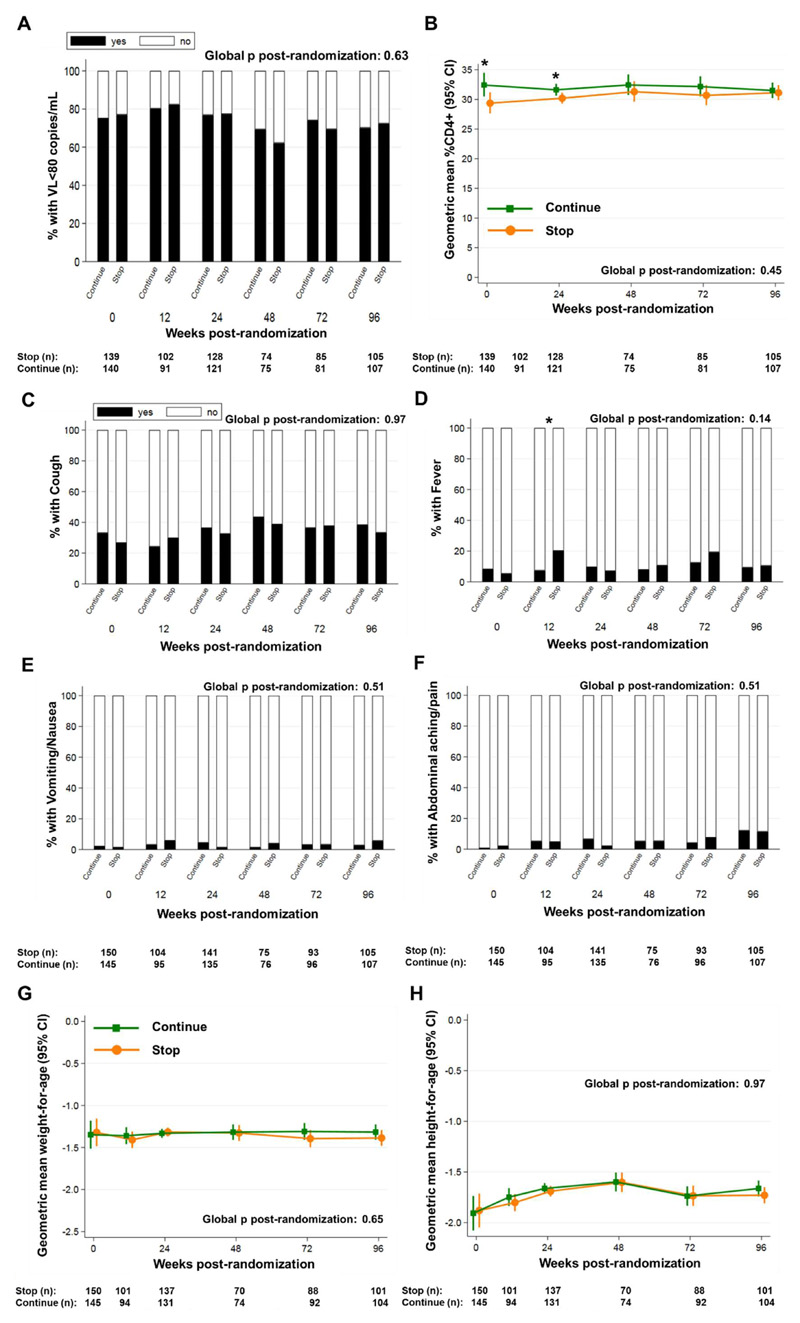
Figure 2. Cotrimoxazole effects on systemic inflammation are not solely due to differences in HIV disease progression, symptomatic infections, or nutritional status.
(A) Percentage of children with viral load <80 copies/mL; (B) geometric mean percentage CD4+ T cells; mean proportions of children with caregiver-reported (C) cough, (D) fever, (E) vomiting/nausea and (F) abdominal pain; geometric mean (G) weight-for-age and (H) height-for-age Z-scores in children randomized to continue versus stop cotrimoxazole prophylaxis (n per group shown under each graph). Randomized groups were compared by generalized estimating equations across timepoints (global p) and at individual timepoints using standard regression models (binomial distribution for viral load; normal distribution for log-transformed values) adjusted for recruitment center; *p<0.05, **p<0.01 ***p<0.001.