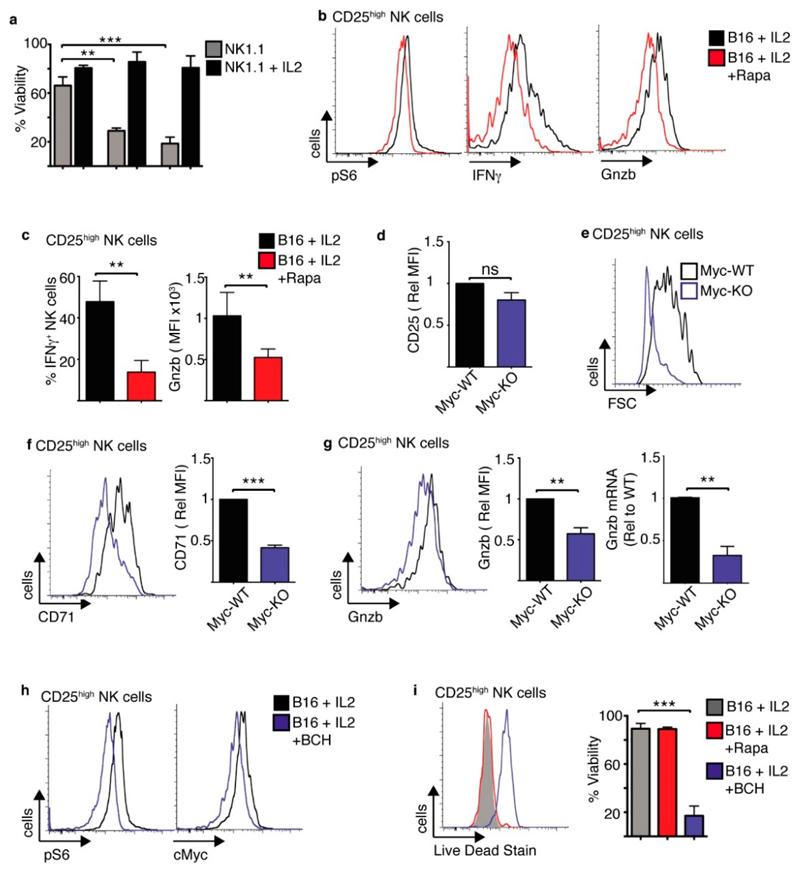
Figure 8. mTORC1/cMyc promote the function and survival of tumour interacting CD25high NK cells.
(a) Cultured NK cells (3 days in IL15 10 ng/mL) were purified and stimulated with a plate bound α-NK1.1 antibody (10 μg/mL) ± IL2 (20 ng/mL) for 24–72 h and analysed by flow cytometry for cell viability. (b–c) Cultured NK cells (6 days in IL15 10 ng/mL) were purified and then co-cultured with B16 melanoma cells or for 18 h, washed and put back into culture with IL2 (20 ng/mL) ± rapamycin for 24 h before analysis of CD25high NK cells by flow cytometry for phosphorylated S6 ribosomal protein (pS6), IFNγ production and granzyme B expression. (d–g) Cultured (6 days in IL15 10 ng/mL) from cMycfl/fl Tamox-Cre and cMycwt/wt Tamox-Cre were treated with tamoxifen (0.6 μM) and then co-cultured with B16 melanoma cells with IL2 (20 ng/mL) for 18 h before analysis by flow cytometry for cell size and the expression of CD25, cell size, CD71, granzyme B and granzyme B mRNA by rtPCR (g). (h,i) Cultured NK cells (6 days in IL15 10 ng/mL) were purified and then co-cultured with B16 melanoma cells or for 18 h, washed and put back into culture with IL2 (20 ng/mL) ± BCH (25 mM), ± rapamycin (20 nM) as indicated for 24 h before analysis of CD25high NK cells by flow cytometry for phosphorylated S6 ribosomal protein (pS6), cMyc and cell viability. Data is representative (b,e,f,g,h,i) or mean ± SEM (a,c,d,f,g,i) of 3–5 independent experiments. Data was analyzed using a one way ANOVA with a tukey post test or a paired students t-test. (** p < 0.01, *** p < 0.001).