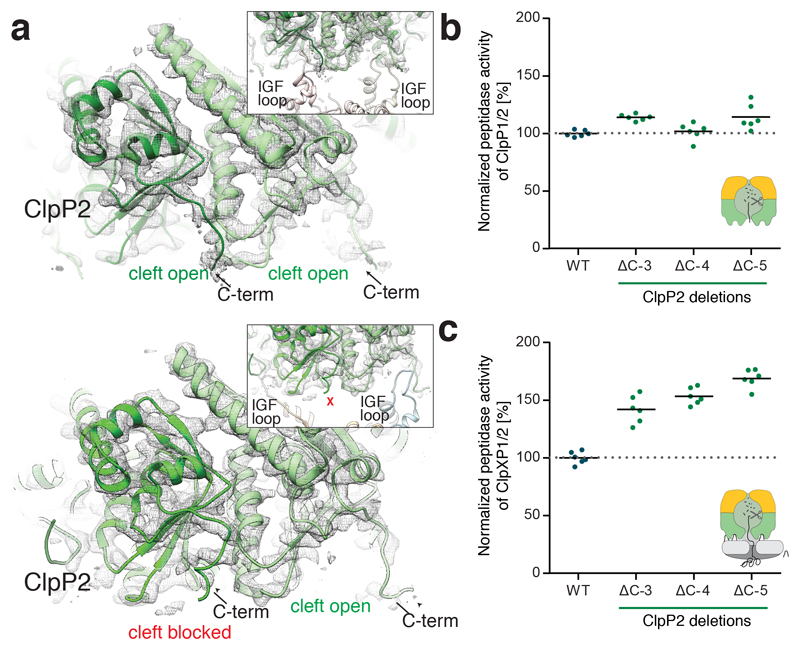
Figure 5. Role of the ClpP2 C-terminus in ClpXP1/2 binding.
a) Molecular model and cryo-EM density of IGF-loop bound (upper image) and not bound to hydrophobic pockets of ClpP2 (lower image). The insets show the respective IGF-loops in ribbon representation. Arrows indicate the C-terminus of ClpP2. b) Peptidase activity of ClpP1/2 with C-terminally truncated ClpP2 (714 nM (ClpP1/2)14, 100 μM Ac-Ala-hArg-2-Aoc-ACC). c) Protease activity of ClpXP1/2 with C-terminally truncated ClpP2 (0.2 μM (ClpP1/2)14, 0.4 μM ClpX6, 0.8 μM GFP-SsrA). Data are normalized to the wild type as 100% (n = 6, data were recorded in triplicate and two independent experiments were performed, black lines denote means). Source data for graphs in b-c are available online.