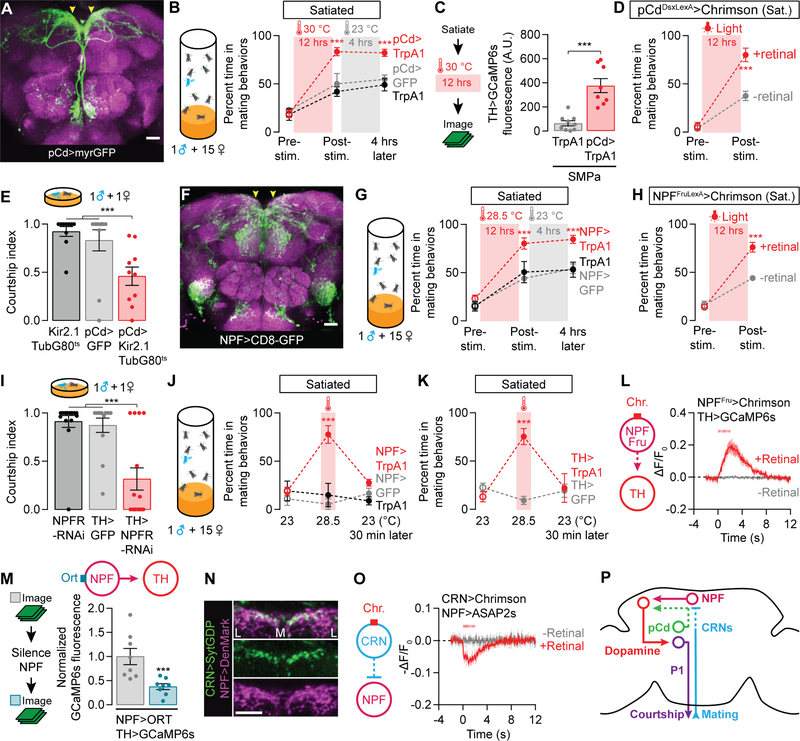
Figure 2. pCd and NPF neurons recharge mating drive after satiety.
(A–B) Prolonged thermogenetic stimulation of pCd neurons (A) accelerates the recovery from satiety, an effect that lasts long after the stimulation ceases (B) (two-way ANOVA, n = 4–8 groups of 5–7 males). Arrowheads point to the SMPa. See Figure S2A for data using other Dsx-related Gal4 lines. (C) Thermogenetic stimulation of pCd neurons accelerates the recovery of calcium levels in dopaminergic projections at the SMPa (t-test, n = 10–11 male brains). See Figure S2C for sample images and Figure S2D for fluorescence data from an unrelated brain region. (D) Prolonged optogenetic stimulation of Dsx+ pCd neurons speeds the recovery of mating drive (flies that have not been fed the obligate chromophore retinal serve as a control group; two-way ANOVA, n = 5 groups 5–7 males each). (E) Conditional, long-term silencing of pCd neurons in adult males decreases courtship (one-way ANOVA, n = 10–11 males). (F–H) Prolonged thermogenetic stimulation of all NPF neurons (G) or the Fru+ subset (H) that projects to the SMPa (arrowheads in F, also see Figure S2K), accelerates recovery from satiety (two-way ANOVA, G: n = 5–6 groups of 5–7 males, H: n = 5 groups). (I) Knockdown of NPFR in dopaminergic neurons decreases courtship (one-way ANOVA, n = 13–16). (J–K) Short-term (20 min) thermogenetic stimulation of NPF (J) or dopaminergic (K) neurons reverts satiety, but this effect is time-locked to the stimulation (two-way ANOVA, J: 5–8 groups of 5–7 males, K: n = 5–6 groups). (L) Optogenetic stimulation (8 ms pulses, 15.5 Hz for 2 s) of the Fru+ NPF neurons excites dopamine neurons projecting to the SMPa (n = 5–7 male brains). (M) Chemogenetic silencing of NPF neurons reduces the baseline calcium activity in dopaminergic projections to the SMPa (one-way ANOVA with data in Figures S3A–S3B, n = 8). See Figures S3A–S3B for controls and pCd-silencing experiments. (N) The ascending axons of the CRNs terminate near NPF dendrites in the SMPa. Letters L and M delineate the medial-lateral axis. (O) Optogenetic stimulation (8 ms pulses, 15.5 Hz for 2 s) of the CRNs causes hyperpolarization of the NPF projections at the SMPa (n = 5–8 male brains). (P) The CRNs decrease mating drive through inhibition of NPF neurons. See also Tables S1 and S2.