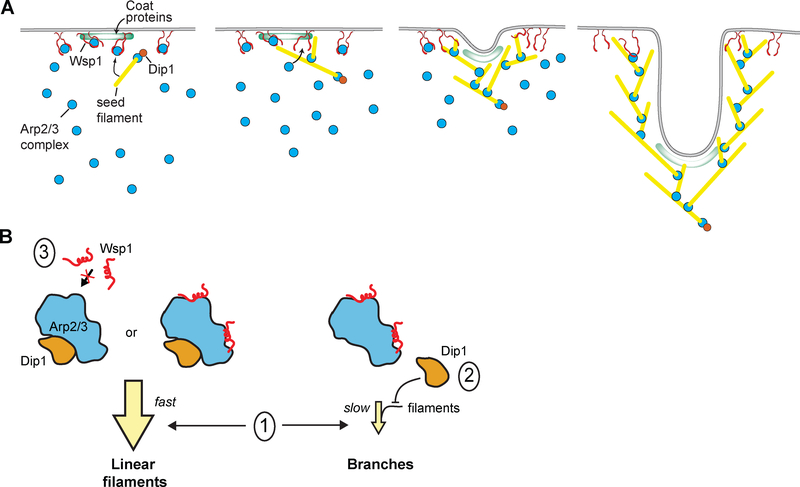
Figure 4: Proposed models of Dip1 activity and dynamics in vitro and at endocytic patches.
A. Cartoon model showing activity of Dip1 (orange circle), Wsp1 (paired red lines), Arp2/3 complex (cyan circles), coat proteins (green zone) and actin (yellow lines) during membrane invagination of an endocytic patch. The number of molecules depicted is roughly proportional to the measured concentrations of these proteins at endocytic sites [12,31]. Dip1 activates Arp2/3 complex to create a linear filament that initiates assembly of the actin network. Dip1 remains bound to Arp2/3 complex so it can only activate a single Arp2/3 complex during assembly of the network. Most Wsp1 molecules remain bound to the cortex allowing them to catalyze multiple rounds of branching. The Dip1-bound linear filament that primed network assembly moves inward as Wsp1-mediated activation of the complex nucleates branches at the cortex. B. Cartoon model showing potential mechanisms of coordinate Arp2/3 complex regulation by Dip1 and Wsp1. The three Arp2/3 complexes represent the different possible binding states of the complex in the presence of both NPFs. Given that WDS proteins have been shown to block actin filament binding, we assume that Arp2/3 complex bound to both activators would create a linear actin filament [16]. Model l: faster kinetics of activation of the Arp2/3 complex by Dip1 compared to Wsp1-bound Arp2/3 complex. Model l explains the dominance of Dip1 over Wsp1 in vitro. Model 2: competition between Dip1 and actin filaments for binding to Arp2/3 complex. Our data indicate that model 2 does not contribute to the dominance of Dip1 over Wsp1 in vitro, though this competition may be important in vivo. Model 3: competition between Dip1 and Wsp1 for binding to Arp2/3 complex. Our data argue against a role for model 3 either in vitro or in vivo.