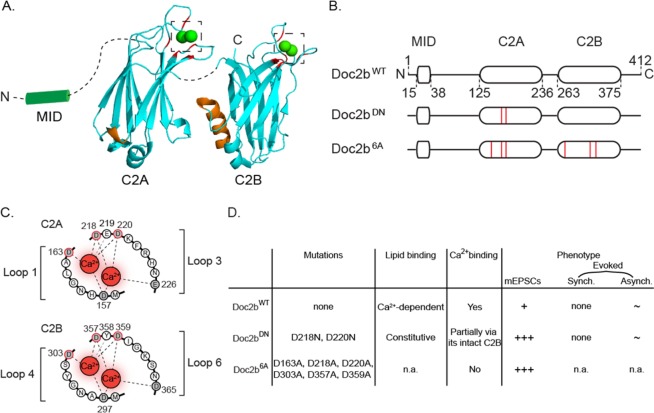
Figure 1.
Molecular and phenotypic properties of Doc2b and its Ca2+-binding site mutants. (A) Cartoon showing C2 domain structures of Doc2b based on crystallography51. Aspartates involved in Ca2+ binding are marked in red; poly-lysine sequences for SNARE complex and PIP2 interaction are marked in orange66. Note that the poly-lysine region is oriented opposite to the Ca2+-binding aspartates. Dashed lines represent linker sequences between domains. Dashed squares highlight Ca2+-binding pockets enlarged in C. (B) Linear representation of Doc2bWT and two previously investigated mutants Doc2bDN and Doc2b6A (red lines indicate amino acid substitutions). (C) Aspartates substituted in Doc2bDN (D218, 220N) or Doc2b6A (D163, 218, 220, 303, 357, 359A). (D) Summary of functional effects of Doc2bDN and Doc2b6A mutations. Ca2+-binding capacity was assessed by tryptophan fluorescence measurements49 for Doc2b6A and isothermal titration calorimetry (ITC) measurement for Doc2bDN (termed CLM mutant)44. Synaptic release phenotypes were determined by electrophysiology in cultured neurons. Doc2bWT supports spontaneous release (marked as ‘+’ in the table). Its role in asynchronous release is observed in some but not all systems29,44,47 (marked as ‘~’). It does not function in synchronous release (‘none’). Doc2bDN constitutively binds phosphatidylserine-containing membranes44,48 and partially binds Ca2+ via its intact C2B domain44. It increases spontaneous release frequency (+++)21,29 and is implicated in asynchronous release in some but not all studies (~)21,44. No effect was noticed on synchronous release (none). Doc2b6A was shown have an abrogated Ca2+-binding29 capacity but its lipid association activity was not reported (n.a.). This mutant increased spontaneous release frequency29 (+++) but its effect on evoked synchronous and asynchronous release were not investigated (n.a.). See21,29,44 for more details.