Abstract
Available evidence indicates that a therapeutic drug monitoring strategy leads to major cost savings related to the anti‐tumour necrosis factor‐α therapy in both inflammatory bowel disease and rheumatoid arthritis (RA) patients, with no negative impact on efficacy. However, although the systematic use of therapeutic drug monitoring could potentially be beneficial and economically acceptable to drug dose optimization, it is not justifiable for all drugs. Infliximab (IFX) is a chimeric monoclonal immunoglobulin G1 targeting tumour necrosis factor. It has been approved for the treatment of immuno‐inflammatory diseases, including RA, ankylosing spondylitis, psoriatic arthritis, Crohn's disease and ulcerative colitis. IFX's pharmacokinetics is highly variable and influences clinical response in chronic inflammatory diseases. Clinical response increases with IFX trough concentrations in RA, ankylosing spondylitis, inflammatory bowel disease and psoriatic patients. Target concentrations predictive of good clinical response were proposed in RA, Crohn's disease and ulcerative colitis. The purpose of this article is to review the current literature surrounding IFX serum concentrations and their related parameters with disease activity in patients with spondyloarthritis. Gathering information about the efficacy of IFX in patients with spondyloarthritis and relating IFX serum concentrations to disease activity were the main goals of this study.
Keywords: ankylosing spondylitis, infliximab, pharmacokinetics, spondyloarthritis, therapeutic drug monitoring
What is already known on this subject
This is a review about the use of infliximab (IFX) drug monitoring in patients with spondyloarthritis (SA). There are very few conclusive studies evaluating IFX serum levels in patients with SA.
What this paper adds
The purpose of this article is to review the current literature surrounding IFX serum levels and related parameters with disease activity in patients with SA. Gathering information about the efficacy of IFX in patients with SA and relating IFX serum concentrations to disease activity were the main goals of this study.
1. INTRODUCTION
The use of a monoclonal antibody against tumour necrosis factor (TNF)‐α has changed clinical practice in chronic inflammatory diseases where this cytokine is involved. However, despite this substantial progress in the treatment of rheumatic diseases, such as rheumatoid arthritis (RA) and spondyloarthritis (SA), only 60–70% of patients with these diseases achieve a long‐term clinical response.1 This treatment presents several critical issues related to primary and secondary failure of TNF antagonists in patients with inflammatory rheumatic diseases, including the possible linkage of anti‐drug antibodies (ADA) and the low serum drug concentrations.2, 3, 4
Therapeutic drug monitoring (TDM) is defined as “a multi‐disciplinary clinical speciality aimed at improving patient care by individually adjusting the dose of drugs for which clinical experience or clinical trials have shown improved outcome in the general or special populations”.5 It is increasingly used to improve disease outcomes in rheumatic diseases. To understand how useful the drug dose is in clinics, it is always necessary to have knowledge of the relation between concentrations and effects. However, although the systematic use of TDM could be potentially beneficial and economically justified leading to optimization of drug dose,6, 7 it is not justified for all drugs and disorders. However, available evidence indicates that a TDM strategy leads to major cost savings related to anti‐TNF therapy in both inflammatory bowel disease (IBD) and RA patients, with no negative impact on efficacy.8 Consequently, to demonstrate that this tool could be useful in a clinical field, it is necessary to understand the relation between serum concentrations and therapeutics effects.
In this way, since a difference in TNF concentrations between immune‐inflammatory diseases could lead to differences in pharmacokinetics (PK), it is necessary to study each disease individually and establish TDM depending on the basal disease.9, 10, 11 Moreover, as a result of an antigenic burden variation (associated with disease activity) and the presence of ADA, there is a highly intra‐ and interindividual variability in serum anti‐TNF drugs concentrations.12 Besides, methotrexate can influence anti‐TNF clearance and lead to increased anti‐TNF concentrations, partly by ADA formation.12 Body weight also seems to influence these PK drugs, as volume of distribution and/or clearance increases with body size. Finally, serum albumin seems to have an inverse relationship with anti‐TNF drugs clearance.12
Infliximab (IFX) is 1 of the most used anti‐TNF drug for the treatment of RA, SA and IBD. The influence of serum IFX concentrations on the maintenance of efficacy has been reported in studies with inflammatory diseases, such as RA and IBD.10, 11
The purpose of this article is to review the current literature surrounding IFX serum concentrations and related parameters with disease activity in patients with SA. Gathering information about the efficacy of IFX in patients with SA and relating IFX serum concentrations to disease activity were the main goals of this study.
A secondary objective was to analyse if there are factors described that could affect IFX serum concentrations in patients with SA.
2. METHODS
2.1. Search strategy and studies selection
A literature search was performed using MeSH terms and keywords in PubMed, Cochrane Library and EMBASE databases. Search strategy is described in Table 1. Additional articles have been identified by citation tracing, which was carried out a later date.
Table 1.
Search strategy
| #1. (Infliximab [MeSH Terms]) AND (1970/01/01″[Date ‐ Publication]: “2018”[Date – Publication) |
| #2. Spondyloarthropathies [MeSH terms] |
| #3. (drug monitoring [MeSH terms]) OR pharmacokinetics [MeSH terms] OR serum trough levels) OR serum trough concentrations) OR serum level) OR serum concentration) OR drug level) OR drug concentration)) |
| #4. (Spondyl*) (* = truncation symbol) |
| #5. (“drug monitoring”) OR pharmacokinetic) OR “serum trough level”) OR “serum trough concentration”) OR “serum drug concentration”) OR “trough level measurement”) OR “serum level”) OR “serum concentration”) OR “drug level”) OR “drug concentration” |
| #6. #1 AND #2 AND #3 |
| #7. #1 AND #4 AND #5 |
| #8. #6 OR #7 |
Article selection and identification in the databases were independently and systematically performed by the authors who carried out initial identification through the title and the abstract. Then, relevance and eligibility criteria were reviewed. Later, a list of potentially relevant full text articles was created and reviewed for relevance. They were essential to meet the provisional, intentionally overly inclusive, eligibility criteria to reduce the risk of inappropriate exclusions by a single reviewer. Discrepancies were solved through consensus among authors.
2.2. Study eligibility criteria
We performed a manual selection of studies that satisfied the essential criteria: the inclusion of data from IFX serum concentrations in patients with spondyloarthritis. From these studies, other data needed for the review purpose were identified and collected: disease activity, possible factors that affect serum concentrations (such as human leucocyte antigen [HLA]‐B27, C‐reactive protein, concomitant use of other immunosuppressive drugs) and the appearance of ADA. Studies in languages other than Spanish or English or those whose full text could not be found were excluded.
3. RESULTS
3.1. Characteristics of included studies
Searches identified 168 articles, of which 23 duplicates were discarded. After the initial title and abstract screening, 22 full‐text studies were assessed for eligibility. After revision, studies without data (n = 11) and 4 reviews were excluded. Thus, 7 articles met the inclusion criteria and 5 articles were added after citation tracing. Figure 1 shows a flow diagram.
Figure 1.
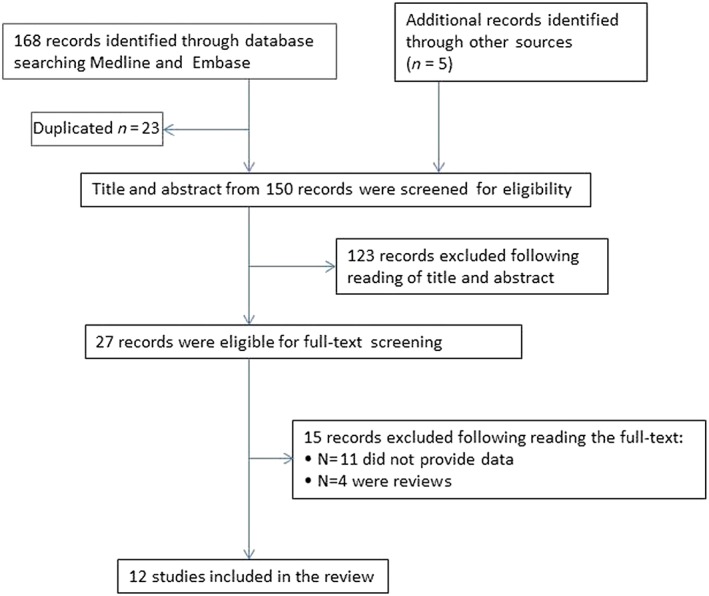
Flow chart of the systematic review
Most of these are about ankylosing spondylitis (AS) and IFX treatment (8/12),13, 14, 15, 16, 17, 18, 19, 20 but 4 studies also evaluated different SA.21, 22, 23, 24 Three articles studied other anti‐TNF drugs in addition to IFX.21, 22, 23
Details about study design, patients and IFX treatment on all included articles are described in Table 2, except the study by Hernandez Flórez et al15 as noninteresting data were provided. The main aim of this study was to determine whether quantitative or qualitative assay data provide accurate information on the assessment of IFX concentrations in AS. Then, IFX concentration data were shown graphically by box‐plots (non‐numeric data) while following the kit manufacturer's guidelines of 2 different kits. Therefore, the authors only showed that the qualitative agreement was better than the quantitative agreement.
Table 2.
Results from all the included studies
| Reference | Study design | Patients (n) | IFX treatment | Serum IFX concentration Cmin and/or Cmax (μg/mL) | Disease activity by rate of patients over time achieved a response |
|---|---|---|---|---|---|
| Studies only in ankylosing spondylitis | |||||
| Kobayashi, 201714 | Multicentre open‐label study from December 2006 to August 2010. | 33 patients | Dose: 5 mg/kg at 0, 2, 6 and every 6 weeks up to 48 weeks. After 50 weeks every 8 weeks. | Cmin (median), Cmax (median) | ASAS20 |
| Week 0: <0.10, 86.28 | Week 2: 69.7% | ||||
| Duration (mean): 149.5 weeks. | Week 2: 23.53, 115.02 | Week 6: 90.9% | |||
| Week 6: 16.76, 115.00 | Week 24: 97.0% | ||||
| Week 12: 8.41, 102.27 | BASDAI 20 | ||||
| Week 18: 7.23, 101.90 | Week 2: 78.8% | ||||
| Week 24: 7.01, – | From week 6: >90% | ||||
| Week 48: 7.37, – | BASDAI 50 | ||||
| Week 50: 2.77, – | Week 2: 39.4% | ||||
| Week 98: 6.15, – | From: ≥75% | ||||
| Week 122: 6.96, – | BASDAI 70 | ||||
| 6 weeks after last infusion: 6.53, – | |||||
| Week 2: 21.2% | |||||
| 16 weeks after last infusion: <0.10, – | From week 24: ≥50% | ||||
| BASDAI score improved to ≤ 3 | |||||
| Week 24: 75.8% | |||||
| Week 48: 81.3% | |||||
| From week 98: >90% | |||||
| BASMI | |||||
| Week 24: 54.5% | |||||
| BASFI score improved to ≥ 2 | |||||
| Week 24: 63.6% | |||||
| Week 48: 68.8% | |||||
| Week 98: 71.4% | |||||
| Week 122: 69% | |||||
| Park, 201316 | Randomized, double‐blind, multicentre, parallel group study from November 2010 to December 2011. | IFX group: 125 patients | Dose: 5 mg/kg at 0, 2, 6 weeks and then every 8 weeks. | Cmin (median), Cmax (median) | ASAS 20 |
| Week 0: 29.8, 145.3 | Week 14: 64,8% | ||||
| Week 30: 72,4% | |||||
| Week 2: 22.8, 181.4 | ASAS 40 | ||||
| Week 6: 7.1, 166.3 | Week 14: 45.9% | ||||
| Week 14: 4.8, 153.6 | Week 30: 47.4% | ||||
| Week 22: 3.6, – | |||||
| Week 30: –, 147.8 | |||||
| CT‐P13 group: 125 patients | Cmin (median), Cmax (median) | ASAS 20 | |||
| Duration: 30 weeks. | |||||
| Week 0: 29.1, 155.8 | Week 14: 64.8% | ||||
| Week 2: 20.1, 175.6 | Week 30: 72.4% | ||||
| Week 6: 6.9, 172.3 | ASAS 40 | ||||
| Week 14: 4.5, 158.4 | |||||
| Week 14: 41.7% | |||||
| Week 30: 51.8% | |||||
| Week 22: 4.2, – | |||||
| Week 30: –, 152.8 | |||||
| Chu Miow Lin, 201018 | Prospective observational study. | There were 22 patients in long‐term maintenance with IFX | Dose (median): 5.0 (range 4.2–7.8) mg/kg | At baseline, median Cmin (range): 6.4 (0.0–20.7). | Median BASDAI was assessed at each infusion for 52 weeks (range): 3.3 (0.4–8.0). |
| Time elapsed since previous infusion: 6.4 (5.1–17.4) weeks. | Patients with an IFX concentration above the media showed a low risk of treatment failure (P = .14). | ||||
| Duration: 30.2 (8.3–52.3) mo. | |||||
| Krzysiek, 200913 | Prospective, randomized multicentre study. | Q6 group: 93 patients | Q6: Continuous treatment group. | Median Cmin (range) | ASAS20 |
| Week 6: | |||||
| Dose: 5 mg/kg at 4, 6, and 10 weeks, and then every 6 weeks. | Week 6: | Responders (61/89): 68.5% | |||
| Respondersa: 24.5 (15.1–33.3) P = .014. | Nonresponders (28/89): 31.5% | ||||
| Nonresponders: 32.5 (26.3–38.3). | Week 10: | ||||
| Week 10: | |||||
| Responders (63/89): 70.8% | |||||
| Duration: 52 weeks. | Nonresponders (28/89): 31.5% | ||||
| Responders: 17.8 (9.2–26.4) P = .05. | Week 16: | ||||
| Nonresponders: 22.4 (18.0–32.1). | |||||
| Responders (62/89): 69.7% | |||||
| Nonresponders (28/89): 31.5% | |||||
| Patients with disease outbreaks in 2 consecutive visits received doses of 7.5 mg/kg starting at week 40. | Week 16: | Week 46: | |||
| Responders: 10.1 (4.1–16.2) P = .064. | Responders (57/89): 64.0% | ||||
| Nonresponders: 16.0 (7.5–22.2). | Nonresponders (20/89): 22.5% | ||||
| Week 46: | Week 52: | ||||
| Responders: 7.7 (4.0–14.3) P = .986. | Responders (62/89): 69.7% | ||||
| Nonresponders: 8.6 (1.7–14.3). | Nonresponders (22/89): 24.7% | ||||
| Week 52: | |||||
| Responders: 7.1 (4.0–12.3) P = .923. | |||||
| Nonresponders: 10.1 (0.4–15.7). | |||||
| On demand group: 76 patients | Dose: 5 mg/kg at 4, 6, and 10 weeks, and then only upon relapse, with a minimum interval of 4 weeks between 2 infusions. | Week 6–10: Similar results to those observed in the Q6 group, with no difference between the patients MTX+/−. | At relapse the serum IFX concentrations were closely correlated with the moment of relapse: the earlier the relapse, the higher the serum IFX concentration. | ||
| At the time of relapse: | 65 patients experienced relapse: | ||||
| 24 (36.9%) had a resurgence of clinical symptoms at a serum IFX concentration of >10 μg/mL. | |||||
| Duration: 54 weeks. | Week 16 or before: | ||||
| 25 (38.4%) experienced a relapse at a concentration of <0.5 μg/mL. | |||||
| MTX+/− did not differ in terms of the moment of relapse or IFX concentration at this moment. | |||||
| Randomly allocated to MTX+/−. | MTX+ (5/65): 25.5 (17.3–42.9) P = .025. | ||||
| MTX– (16/65): 4.2 (0.4–11.2). | |||||
| Total (24/65): 15.8 (6.8–29.3). | |||||
| Week 17–22: | |||||
| MTX+ (9/65): 4.2 (0.4–11.2). | |||||
| MTX– (13/65): 1.6 (0.5–9.5). | |||||
| Total (22/65): 2.71 (0.39–11.16). | |||||
| Week 23 or after: | |||||
| MTX+ (12/65): 0.4 (0.2–0.7). | |||||
| MTX– (7/65): 0.3 (0.2–0.4). | |||||
| Total (19/65): 0.3 (0.2–0.6). | |||||
| Ducourau, 201119 | Retrospective study from December 2005 to January 2009. | 91 patients | Dose: 5 mg/kg at 0, 2, 6, 12 weeks and every 6 weeks. | Median Cmin (range) | Not specified. |
| Week 2: | |||||
| ADA+: 25.0 (4.0–40.7). | |||||
| ADA–: 35.8 (14.3–57.2) P = .03. | |||||
| Week 6: | |||||
| ADA+: 11.9 (0–24.2). | |||||
| ADA–: 29.5 (3.4–69.0) P < .001. | |||||
| Week 12: | |||||
| ADA+: 1.6 (0–13.5). | |||||
| ADA–: 15.8 (0.7–47.3) P < .001. | |||||
| Meric, 201117 | Observational open label study from May to August 2007. | 32 patients | Median dose (range): 5 mg/kg 13, 25, 26, 27, 28. | Median Cmin (range): 4.79 (<0.014 to 29.27). | Median BASDAI (range): 3.66 (0.0–7.39). |
| Interval between IFX infusion: 7 (6–13) weeks. | According to available published data criteria: | 9 patients (28%): optimal control (BASDAI <2). | |||
| 11 patients: High IFX concentration (≥8.0) | |||||
| Duration: 31 (4–75) mo. | 11 patients: Medium IFX concentration (≥2.0–< 8.0) | ||||
| 10 patients: Low IFX concentration (<2.0) | 12 patients (38%): Acceptable control (BASDAI 2–4). | ||||
| 11 patients (34%): Inadequate control (BASDAI >4). | |||||
| De Vries, 200720 | Prospective observational study. | 38 patients | Dose: 5 mg/kg at 2, 6 weeks and every 6 weeks. | Cmin (mean) | Median BASDAI |
| Week 54: | Baseline: 6.4. | ||||
| Week 24: 3.6 (P < .001) | |||||
| Respondersa (21/38): 8.2. | |||||
| In case of decrease of clinical response, the dose was increased to 7.5 mg/kg. | Nonrespondersa (7/38): 6.3 (P < .018). | Week 54: 4.1 (P < .001) | |||
| ASAS 20: | |||||
| Week 24: 63% responders | |||||
| Week 54: 53% responders | |||||
| Studies in spondyloarthropathies: ankylosing spondylitis, undifferentiated spondyloarthritis, psoriasis arthritis | |||||
| Inciarte, 201621 | Cross‐sectional study with PsA patients in remission or low disease activity included. | 50 patients , only 10 with IFX treatment | Dose: No specified. | Median Cmin (range) | Median DAS28‐ESR (range) |
| Median duration (range): 58.3 (7.6–166) mo | |||||
| PDUS+: 2.86 (0.1–6.5). | PDUS+: 2.15(1.1–3.1) | ||||
| PDUS+: 16 | |||||
| PDUS–: 34 | |||||
| PDUS–: 1.67(1–2.7) P < .05 | |||||
| PDUS–: 3.21 (0.7–7.7). | |||||
| Almirall, 201622 | Cross‐sectional study from June 2014 to December 2014. | 20 patients , only 4 with IFX treatment | Dose: 3 mg/kg every 8 weeks. | Cmin (4 patients) | Not specified |
| Duration (mean ± SD): 7.4 ± 2.5 y. | Therapeutic drug concentrations: 2.12 and 2.7. | ||||
| Nontherapeutic drug concentrations: 0.02 and 1.13. | |||||
| Arstikyte, 201523 | Retrospective observational study from January 2012 to December 2013. | 81 patients , only 33 with IFX treatment | Dose (mean ± SD): 2.59 ± 1.67 mg/kg. | Median Cmin (range): 2.33 (1.69–35.0). | Not specified for IFX treatment only |
| Due to an inadequate response a gradual escalation of IFX dose to 3.98 ± 2.4 mg/kg was given. | Respondersb VS no responders: P = .293. | ||||
| Median duration: 54 (3–108) mo. | |||||
| AS patients: 49 | |||||
| PsA patients: 32 | |||||
| Plasencia, 201224 | Ambispective observational study from 1999 to 2010. | 94 patients | Dose: 5 mg/kg at 0, 2, 6 weeks and then every 8 weeks. | Median Cmin (range) | ASDAS (mean ± SD) |
| 6 mo: | At baseline = 3.08 ± 1.31 without differences between ADA+/−. | ||||
| AS patients: 50 | 19% needed more frequent infusions because the response obtained was inadequate. | Inactive diseasec: 4.99 (2.98–8.77). | 6 mo: | ||
| Moderate: 2.05 (0.84–4.11) P = .001. | |||||
| Und SpA patients: 12 | |||||
| Duration (mean ± SD): 5.9 ± 2 y. | |||||
| PsA patients: 22 | High: 1.10 (0–3.15). | ||||
| SpA‐IBD patients: 10 | |||||
| 1 y: | ADA+: 2.55 ± 0.89. | ||||
| Inactive disease: 4.13 (2.77–7.82). | ADA–: 1.79 ± 1.04. | ||||
| Moderate: 2.34 (0.85–3.57) P = .010. | |||||
| High: 1.33 (0–3.37). | P = .038. | ||||
| >4 y: | |||||
| 1 y: | |||||
| Inactive disease: 4.19 (2.87–5.34). | |||||
| Moderate: 2.43 (0.64–4.29) P = .009. | |||||
| High: 0.31 (0–3.30). | ADA+: 1.95 ± 0.67. | ||||
| ADA–: 1.67 ± 0.71. | |||||
| P = .042. | |||||
| >4 y: | |||||
| ADA+: 2.52 ± 0.99. | |||||
| ADA–: 1.53 ± 0.81. | |||||
| P = .024. | |||||
IFX: infliximab; Cmin: minimum serum concentration; Cmax: maximum serum concentration; ASAS20: ASAS Response Criteria‐20; BASDAI20: Bath Ankylosing Spondylitis Disease Activity Index‐20; BASDAI50: Bath Ankylosing Spondylitis Disease Activity Index‐50; BASDAI 70: Bath Ankylosing Spondylitis Disease Activity Index‐70; BASMI: Bath Ankylosing Spondylitis Metrology Index; BASFI: Bath Ankylosing Spondylitis Functional Index; CT‐P13: IFX biosimilar; MTX: methotrexate; ADA: antidrug antibodies; PsA: Psoriatic arthritis; PDUS: power Doppler ultrasound synovitis; SD: standard deviation; DAS28‐ESR: 28‐joint Disease Activity Score based on erythrocyte sedimentation rate; AS: ankylosing spondylitis; Und SpA: undifferentiated spondyloarthritis; SpA‐IBD: spondyloarthritis associated with inflammatory bowel disease; ASDAS: Ankylosing Spondylitis Disease Activity Score; mo: months; y: year.
Responders were defined as patients fulfilling the ASAS20 criteria at week 58.
AS patients with inactive disease or moderate disease activity were attributed to responders while patients with high or very high disease activity were considered as nonresponders.
Inactive: ASDAS<1.3, moderate: ASDAS ≥1.3 and < 2.1, high: ASDAS ≥2.1 and ≤ 3.5, and very high: ASDAS >3.5.
Analysing the data detailed in the table regarding posology, all studies used a dose of 5 mg/kg except those by Almirall et al22 and Arstikyte et al,23 that used 3 mg/kg, and by Inciarte‐Mundo et al21 where the dose was not specified. Inciarte‐Mundo et al21 only described that some patients were receiving reduced dose of biological therapy due to persistent remission and/or low disease activity. The interval between doses is also different; some studies used 6 weeks while others used 8.
Effectiveness evaluation was indicated with different indexes, both for quality of life and clinical activity, which were available for each studied pathology; in the table, we specified articles we considered most relevant, or those that are related to IFX serum concentrations. Not all studies included activity data. In those that did, different activity and quality of life scores were used.
3.2. Infliximab pharmacokinetics
In general, the articles found in our search did not refer to details regarding the IFX PK itself. In relation to concomitant drugs influencing IFX concentrations, Krzysiek et al26 (93 patients with AS, treated with IFX) infer that patients who received methotrexate (MTX) and those who did not receive MTX did not differ in terms of the moment of relapse or the IFX concentration at that moment. However, in the subgroup of patients experiencing a relapse before week 16, the serum IFX concentration at the time of relapse was higher in patients receiving MTX. In addition, results of Plasencia et al.’s study24 (94 patients with SA treated with IFX) suggested that the maximum IFX concentrations tended to be higher in patients with concomitant MTX treatment.
3.3. Assays to measure IFX and ADA concentrations
Many techniques have been developed for anti‐TNF drugs and antibody concentration determination. The enzyme‐linked immunosorbent assay (ELISA) technique is often the preferred analysis method because of the low cost and high throughput. Serum IFX concentrations in our search were determined via ELISA, except in the study by Park et al,16 where they were determined by a flow‐through immunoassay platform. Antibody concentrations were measured with radioimmunoassay,20 electro‐chemiluminescent immunoassay method16 and ELISA technique.14, 15, 17, 18, 19, 22, 23, 24 They were not determined in the other studies.13, 21
3.4. Association between IFX and ADA concentrations with clinical response
Serum IFX concentrations were determined immediately before the following administration of anti‐TNF (Cmin) in all studies and another concentration (Cmax) was determined in 2 of them at 1 hour after completion.14, 16 Moreover, depending on the study, the determination was performed during treatment induction and/or treatment maintenance.
Only 6 studies in AS described results about serum IFX concentrations and disease activity; 4 were observational studies14, 17, 18, 20 and 2 were randomized clinical trials.13, 16 Furthermore, only 2 studies in SA reported data about disease activity.21, 23 Detailed data about IFX concentrations and disease activity were shown in Table 2.
The observational study by Kobayashi et al14 provided detailed data about serum IFX concentrations and disease activity. The study included data from induction, maintenance phase and weekly data. The other observational studies17, 18, 20 provided fewer data since they only indicated a median serum concentration and activity index.
De Vries et al20 showed that high concentrations of serum IFX are correlated with a good clinical response and only 5% of responders showed ADA with undetectable IFX concentrations; however, in this small sample no clear increase in serum trough IFX concentrations after dose escalation was shown.
The study of Chu Miow Lin et al18 included patients in long‐term maintenance with IFX (patients at initiation of treatment were excluded from the study). They concluded that IFX concentration in patients with SA seems to predict sustained efficacy of the same IFX regimen throughout treatment. By contrast, Méric et al17 presented a study with 32 patients and concluded that knowledge of IFX trough concentration did not improve the control of disease activity as estimated by the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). IFX dose alterations led to nonsignificant changes in diseased activity, in particular in patients whose IFX dosage was increased. They described that this could be due to 2 factors; firstly, the small number of patients in the study (especially those with an increase in IFX dosage) may explain the lack of statistical power. Secondly, the increase in IFX concentration after dose augmentation may have been too small, which showed that patients with concentrations >6 μg/mL experienced an improvement in their BASDAI.
The 2 randomized clinical trials13, 16 were designed with a different objective. The PLANETAS study16 compared IFX PK safety and efficacy with a biosimilar (CT‐P13) in patients with AS. The study by Krzysiek et al13 compared IFX concentration with the presence of clinical symptoms in patients continuously treated with IFX or after treatment interruption (on‐demand treatment).
Published PK data for the 5 mg/kg dose of IFX in SA is scarce. However, the area under the curve and Cmax values reported in PLANETAS study16 were similar to those reported in IFX monotherapy studies using a similar dosing pattern in Crohn's disease. However, data on serum concentrations and response to treatment can only be indirectly related, as the trial objective was different and did not differentiate the serum concentrations of responders and nonresponders. Specifically, in week 14 of treatment, the average Cmin was 4.8 μg/mL (every 8 weeks interval) and the Assessment of SpondyloArthritis International Society response criteria (ASAS) 20 was achieved in 64.8% of the patients.16
In the study by Krzysiek et al,13 data for the group of patients with 5 mg/kg dose and interval IFX infusion every 6 weeks revealed that treatment failure is not usually explained by insufficient concentrations of circulating IFX and that, conversely, treatment success can be achieved even in patients with relatively low circulating IFX concentrations. Thus, after the induction phase, both responders and nonresponders presented, at weeks 16, 46 and 52, a median Cmin without statistically significant differences. The highest concentrations were observed during the first weeks of treatment, and then they decreased to reach a median steady‐state concentration of 6.7 μg/mL at the end of the follow up period. From the 28 nonresponders, 11 (39.2%) had IFX concentrations of >10 μg/mL at week 52. In contrast, 9 (13.8%) of the 65 responders had a serum IFX concentration of <1 μg/mL. The on‐demand treatment group indicates that, in SA patients, the persistence of an initially controlled short course of treatment is dependent on circulating IFX concentrations. However, the heterogeneity also indicated that the minimal IFX concentration needed to control the disease markedly differs between individuals. In fact, many patients (25 of 65) experienced disease control until their serum IFX concentration fell below 0.5 μg/mL.
In several studies,19, 21, 22, 23 anti‐TNF concentrations were analysed in patients with different types of rheumatological inflammatory disease, so the sample size for IFX was small. The results in general showed a tendency towards higher IFX concentrations in all patients responding to treatment, but the data was not statistically significant.
3.5. Influencing factors on IFX/ADA concentrations and clinical response
There is evidence that different factors related to the patient, disease and drug affect its serum concentrations.29, 30, 31, 32 Below are those that have been described in the studies included in this review (Table 3). Table 3 shows the percentage of patients that developed ADA in the different reviewed studies. In general, studies where antibody concentrations were measured and related to disease activity16, 17, 19, 20, 23, 24 showed that ADA+ patients are associated with loss of clinical response. In addition, other studies showed low IFX serum concentrations in ADA+ patients.14, 18
Table 3.
Antibodies and sensitive biomarkers
| Reference | Patients with antibodies and relation with treatment response | Other biomarkers and factors analysed |
|---|---|---|
| Ankylosing spondylitis | ||
| Kobayashi, 201714 | ADA concentrations: Evaluated at weeks 0, 24, and 50, and at intervals of 24 weeks. Serum IFX and ADA concentrations were also evaluated at week 48, and 6 and 16 weeks after the last infusion. | At baseline: |
| HLA‐B27+: 24 (72.7%). | ||
| Concomitant use of SSZ, MTX or corticosteroids did not markedly influence BASDAI 50 response. | ||
| 6.31% (2/33 patients) ADA+, with low IFX serum concentrations (1 concomitant with MTX and 1 without MTX). | ||
| Any data about ADA and disease activity. | ||
| Park, 201316 | ADA concentrations, evaluated at: | None. |
| Week 14: 11% ADA+ (n = 13). | ||
| Week 30: 22.5% ADA+ (n = 25). | ||
| ADA+ had a less robust ASAS20 response (posthoc analysis). | ||
| Chu Miow Lin, 201018 | At baseline, there were 13.6% (3/22) ADA+ with no detectable IFX concentration. | At baseline: |
| HLA‐B27+: 14 (63.6%) | ||
| Krzysiek, 200913 | Not analysed. | At baseline: |
| HLA‐B27+: Continuous treatment: 69 (79.3%) /on demand: 58 (85.3%). | ||
| Concomitant MTX: Patients who received MTX and those who did not receive MTX did not differ in terms of the moment of relapse (P = .4) or the IFX concentration at that moment (P = .7). However, in the subgroup of patients experiencing a relapse before week 16, the serum IFX concentration at the time of relapse was higher in patients receiving MTX (P = .025). | ||
| Ducourau, 201119 | During follow‐up, ADA concentrations were: | Concomitant MTX: AS patients who received MTX had a lower risk of developing ADA than patients not taking MTX (0 of 14 patients (0%) vs 25 of 77 patients (32%); P = .03). |
| 15.4% (14/91 patients) ADA+: 0 patients MTX+; 14 patients MTX–. | ||
| 84.6% (77/91 patients) ADA–: 25 patients MTX+; 52 patients MTX–. | ||
| Concomitant MTX treatment was lower for ADA+ than for ADA– patients (P = .03). | ||
| This study demonstrates that low trough IFX concentration during treatment initiation is predictive of immunization against IFX on the basis of the presence of ADA. | ||
| Disease duration was longer, but not significantly, for the ADA+ group than for the ADA– group (P = .3). | ||
| Median time of ADA detection after initiation was 3.7 mo (1.7–26). | ||
| Meric, 201117 | ADA+ (IFX serum concentrations below the limit of detection [<0.014 μg/mL]) 18.8% (6/32 patients): | At baseline: |
| HLA‐B27+: 21 (66%). | ||
| ADA+ 6.3% (2/32 patients): Optimal control of disease activity. | ||
| ADA+ 12.5% (4/32 patients): Inadequate control of disease activity. They were in | ||
| The increased dosage decision group. | ||
| De Vries, 200720 | During follow‐up, ADA concentrations were: | At baseline: |
| Week 24: | HLA‐B27+: 32 (84%). | |
| • 7 patients ADA+ (18.4%): 2 responders. | Presence of ADA was significantly associated with the absence of HLA‐B27 (OR = 7.1; 95% CI 1.1–47.6; Pearson's χ2, P = .03). | |
| • 31 patients ADA– (81.6%): 22 responders. | ||
| Week 54: | ||
| • 11 patients ADA+ (29%): 1 responder. | ||
| • 27 patients ADA– (71%): 20 responders. | ||
| Finally, at week 54, 1 responder vs 20 responders (P < .001). | ||
| The absence of ADA remained a significant determinant for ASAS‐20 response, with an OR of 100 (95% CI 5.2 to 1000). | ||
| Spondyloarthropathies: ankylosing spondylitis, undifferentiated spondyloarthritis, psoriasis arthritis | ||
| Inciarte‐Mundo, 201621 | Not analysed | Calprotectin: |
| PDUS+: 2.36 (0.9–4.6) μg/mL (n = 16). | ||
| PDUS–: 0.70 (0.06–3.7) μg/mL (n = 34), P < .05. | ||
| (patients with PDUS synovitis had higher concentrations of calprotectin, CRP and ESR). | ||
| The results of this study show a significant proportion of patients with RA and PsA in remission or with low disease activity, those treated with TNF inhibitors had PDUS synovitis, together with significantly higher concentrations of serum calprotectin, which was more accurate than acute phase reactants in identifying PDUS synovitis. | ||
| Patients with PDUS synovitis had significantly lower TNF inhibitors serum concentrations, and there was an inverse correlation between TNF inhibitors serum concentrations and calprotectin in the 2 diseases (RA and PsA). Therefore, calprotectin and TNF inhibitors serum concentrations may be considered as sensitive biomarkers of synovial inflammation in RA and PsA patients in remission or with low disease activity being treated with TNF inhibitors. | ||
| Almirall, 201622 | None of the patients had ADA in serum. | At baseline: |
| HLA‐B27+: 18 (90%). | ||
| Arstikyte, 201523 | Serum samples were collected once during the treatment course (from January 2012 to December 2013). | At baseline: |
| HLA‐B27+: 60 (74.1%). | ||
| ADA+: 18.2% (6 patients) | ||
| data of ADA concentration: 74.4 (4.89–1440) AU/mL. | No correlation between MTX+ and ADA+ | |
| ADA concentration is higher in nonresponders than in responders (P = .243). | ||
| ADA are associated with loss of clinical response, an increased incidence of infusion reactions and treatment emendation. | ||
| Plasencia, 201224 | ADA appeared in the sixth infusion in most of the patients: Median 44 (IQR 24–55 weeks). | At baseline: |
| HLA‐B27+: 17 (58.6%). | ||
| MTX–: 58 (61.7%). | ||
| Globally, there were 24/91 patients ADA+ (25.5%) all of which had undetectable IFX concentrations. | MTX+: (taking MTX before starting TNFi treatment.): 36 (38.3%). 47 patients (50%) were MTX+ at some time during the study. 36 (38%) were MTX+ before starting IFX. | |
| ADA development occurred more frequently in patients not receiving MTX (P = 0.011). | ||
| • 6 mo: (56 patients) | • 20/58 MTX– (34.5%) were ADA+ | |
| • 4/36 MTX+ (11.1%) were ADA+. In these 4 patients MTX+/ADA+, the appearance of ADA occurred later 70.83 ± 62.2 weeks vs 36.50 ± 16.58 weeks in IFX alone (P = .148). | ||
| ADA–: 78.5% (33) | Moreover, patients with inactive disease had higher IFX concentrations (median, IQR) than those with active disease (inactive: 4992, 2976–8768 ng/ml vs moderate: 2048, 840–4112 ng/ml vs high: 1104, 0–3150 ng/ml, P = .001 at 6 mo). | |
| ADA+: 21.5% (2) Inactive disease: 43.5% ADA– VS 0% ADA+ (P = .001). | ||
| • 1 y: (51 patients) | ||
| ADA–: 80.4% (34) | ||
| ADA+: 19.6% (35) Inactive disease: 31.4% ADA– VS 12.5% ADA+ (P = .091). | ||
| • >4 y:(56 patients) | ||
| ADA–: 78.5% (33) | ||
| ADA+: 21.5% (2) Inactive disease: 48.7% ADA– VS 9% ADA+ (P = .001). | ||
ADA: anti‐drug antibodies; MTX: methotrexate; HLA‐B27: human leukocyte antigen B27; SSZ: sulfasalazine; BASDAI‐50: Bath Ankylosing Spondylitis Disease Activity Index‐50; ASAS‐20: ASAS Response Criteria‐20; AS: ankylosing spondylitis; OR: odds ratio; CI: confidence interval; PDUS: power Doppler ultrasound synovitis; ESR: erythrocyte sedimentation rate; RA: rheumatoid arthritis; PsA: psoriatic arthritis; AU: arbitrary units; TNFi: tumour necrosis factor inhibitor; mo: months; y: year.
By contrast, the concomitant use of immunosuppressive drugs could prevent ADA formation, helping to reach nondecreasing serum concentrations, so the biological drug could continue to exert its therapeutic effect. Analysing the possible influence of concomitant immunosuppressants with IFX in SA patients, different results were found depending on each study. In this way, Kobayashi et al14 indicated that the concomitant use of sulfasalazine, MTX or corticosteroids did not markedly influence BASDAI50 response. Similarly, in the study of Krzysiek et al,13 patients who received MTX and those who did not receive it did not differ in terms of the moment of relapse or the IFX concentration at that moment. On the contrary, Ducourau et al19 and Plasencia et al24 showed that patients who were being treated concomitantly with MTX had a lower risk of developing ADA than patients who were not taking an immunosuppressant.
A probable confounding variable is HLA‐B27. The morbidity rate of SA is closely correlated with the expression of HLA‐B27.36 HLA‐B27 is mainly measured at baseline level13, 14, 17, 18, 20, 22, 23, 24; only de Vries et al,20 from a genetic point of view, correlates the absence of HLA‐B27 with ADA formation, considering that further genetic evaluation will be performed to unravel this interesting observation (Table 3).
3.6. TDM strategies in SA patients
With reference to optimization strategies, the PLANETAS study16 compared the PK, safety and efficacy of IFX and CT‐P13, a biosimilar of IFX, in patients with AS. CT‐P13 and IFX were shown to be equivalent in terms of area under the curve and Cmax in patients with active AS. Clinical efficacy endpoints, including ASAS20 and ASAS40 responses, were highly similar between CT‐P13 and IFX groups. CT‐P13 was well‐tolerated with an immunogenicity and safety profile comparable to that of IFX up to week 30. This could lead to cost savings due to the lower price of biosimilars. No further evidence was found about cost‐effectiveness and TDM strategies in SA patients concretely in our selected articles.
4. DISCUSSION
The PK of monoclonal antibodies is highly variable among patients.37 Several covariates were found to be associated with the variability of these PK parameters.38 TDM is currently increasingly used to improve disease outcomes in inflammatory rheumatic diseases. Balsa et al39 evaluated the prevalence of ADA in patients with RA or SA who experienced secondary failure to anti‐TNF therapy (etanercept, adalimumab [ADL] and IFX) and correlated ADA presence with anti‐TNF concentration.
IFX PK is increasingly used to manage and optimize IFX therapy in chronic inflammatory diseases, especially in IBD and RA patients. There is a clear association between clinical response and IFX trough drug concentrations across the spectrum of rheumatic and other inflammatory diseases treated with IFX.34, 39, 40
However, it is necessary to collect more specific information about the relationship between the serum IFX concentrations and clinical response in other pathologies such as SA, where little is known about the efficacy of IFX treatment in relation to its serum concentrations. Moreover, SA comprises of several heterogeneous diseases, and disease activity indexes such as BASDAI or Ankylosing Spondylitis Disease Activity Score (ASDAS) do not always show the real disease activity, so it could be useful to count on another tool as serum IFX concentrations to evaluate disease activity.
Thus, the main contribution of this study is to provide an overview and potential correlation of serum IFX concentrations and disease activity results in SA. In addition, to analyse factors that could affect IFX pharmacokinetics in these patients. This is the first review that summarizes the available evidence of these data.
Our main conclusion is that the studies compile different clinical data, which are difficult to compare, because different dosage regimens and activity indexes are used, different serum concentration determination times and even the main objectives of these studies are very diverse. In study results, data are not sufficiently clear and there are relevant differences in serum concentrations reached in responding and nonresponding patients among the different studies. From all the studies reviewed, the 1 that contributes a greater degree of evidence is the randomized clinical trial by Krzysiek et al.13 They conclude that responsiveness to IFX treatment is highly heterogeneous among individuals with AS, and this parameter overcomes the circulating IFX concentration to explain treatment success or failure. However, de Vries et al20 reported that clinical response was related to IFX concentration, so exposure to IFX may account for the variability in response. The mean serum by the use of IFX concentration for responders was significantly (P < .01) higher that of nonresponders (8.2 vs 6.3 mg/L); however, the design of this study is not clear, and the number of patients was small.
As is known, immunogenicity refers to the development of antibody response to exogenous/foreign agents; the development of neutralizing antibodies to therapeutic drugs may greatly alter their PK, leading to reduced half‐life and efficacy. In literature, the percentage of patients who develop ADA varies among different autoimmune inflammatory diseases. However, as not all patients treated with anti‐TNF agents develop ADA, immunogenicity seems to be the result of several factors associated with the treatment, patient and external factors.24 , 29
In this way, the development of neutralizing antibodies to the anti‐TNF monoclonal antibodies during IFX treatment is a well‐recognized phenomenon41. ADA has been seen in up to 1/3 of RA and about 25% of SA patients.4, 20, 24 Underexposure to IFX has been shown to increase the risk of ADA development and, hence, of treatment failure in RA.42 The detection of ADA could be helpful in understanding the reason for treatment inefficacy when choosing an appropriate medication. Testing for immunogenicity could become a part of a patient's everyday clinical management.23
In our review, ADA seem to modify PK and increase the clearance of immune complexes, thus reducing the serum concentration of the drug. Moreover, ADA+ seem to be correlated to the absence of final therapeutic action. By contrast, there are patients with ADA presence and undetectable drug concentrations who present low activity of the disease or clinical remission; this is because their pathology is probably a refractory form to the TNF neutralization. Moreover, the presence of ADA is usually accompanied by serum concentrations below the detection limit; this could happen because the type of technique used in most studies (ELISA) does not detect ADA until its concentration exceeds that of the drug. In other chronic inflammatory diseases, such as RA and Crohn's disease, ADA have been seen in 12–44% and in up to 29% of patients respectively.24 Therefore, as some authors suggest, monitoring ADA concentrations should play an important role in avoiding the continuation of ineffective treatment.
When speaking about influencing factors on drug concentrations or clinical response in patients with SA, it is interesting to note that monoclonal antibodies are primarily cleared through proteolytic catabolism via the reticuloendothelial system. Proteolytic clearance is generally related to patient weight, with higher weight subjects having a more rapid clearance.33 Weight has therefore commonly been identified as being predictive of antimonoclonal antibodies clearance. Atypical clearance of monoclonal antibodies can be associated with disease type and severity.33 Rosas et al43 showed that obesity decreases clinical efficacy and ADL concentrations in patients with AS. They reported that patients with body mass index >30 kg/m2 (obese) as opposed to <25 kg/m2 (normal), presented lower blood ADL concentrations, increased ASDAS scores, shorter ADL treatment time and increased BASDAI results.
As monoclonal antibodies are not eliminated by renal or biliary excretion and/or by metabolism, it may be thought that monoclonal antibodies PK are not modified by concomitant drugs. However, the IFX PK is found to be influenced by MTX comedication, through concentrations of higher IFX in the presence of methotrexate, in diseases such as RA.44 Although MTX treatment improves activity disease in rheumatic diseases such as RA45; by contrast, it does not seem to be as clear in AS.
It seems that methotrexate cotreatment does not influence IFX PK in AS patients, probably because the antigen burden is lower in this disease than in RA.46 No sound data have reported an influence of other disease‐modifying antirheumatic drugs (such as azathioprine or prednisolone) on monoclonal antibody PK.47
The absence of HLA B27 remarkably exhibits significant correlation with anti‐IFX formation.20 Likewise, it has only been evaluated by 1 study in AS. Further genetic evaluation should be performed to unravel this observation.
TDM with drug serum concentration measurement and ADA detection could be a useful tool that leads to the individualization of anti‐TNF treatment. Available evidence indicates that a TDM strategy leads to major cost savings related to anti‐TNF therapy in both IBD and RA patients,8 but very few studies evaluated IFX serum concentrations in AS patients. Some authors have concluded that ADA formation in inflammatory diseases may increase the risk of lost response and measuring their concentrations could be useful to consider other more effective therapies.48 Concretely and recently, Bornstein et al49 evaluated the prevalence of immunogenicity of anti‐TNF in axial spondyloarthritis patients and assessed the effect of immunogenicity on drug concentrations and clinical response. They concluded that ADA measurement and drug concentrations in these patients may assist in determining further treatment strategies.
Regarding TDM strategies in SA patients, specifically in switching in patients who failed a first anti‐TNF, there is a study by Plasencia et al50 that analysed whether the clinical response to a second anti‐TNF drug is conditioned by the development of ADA against the first drug in a group of SA patients. They concluded that, similarly to RA, the failure to respond to a first anti‐TNF drug due to the development of ADA predicts a better clinical response to a second biological treatment in SA. The presence of ADA against the first anti‐TNF drug is a determining factor for the response to a second drug. The study of the immunogenicity in biological treatment failure may help predict the response to a second biological treatment in SA. Benucci et al51 studied the real‐life efficacy, safety and immunogenicity of switching from innovator to biosimilar IFX in an Italian cohort of 41 patients with SA. The switch from innovator to biosimilar IFX in that multicentre SA cohort was not associated with any statistically significance differences in efficacy, adverse events or anti‐drug antibody concentrations. This is another example, other than the PLANETAS study,16 where TDM could be useful in cost savings.
5. CONCLUSIONS
In our opinion, the results from included studies are very heterogeneous. Apart from this heterogeneity that highlights the need for focusing on these measurements, there is the impossibility until now of using IFX monitoring as an effective method to assist in the therapeutic decisions in SA. It is necessary to design larger studies, with the doses usually used in these diseases and appropriate administration interval, to prove the relationship between IFX concentrations and the improvement of activity disease in order to determine whether TDM could be considered as a useful tool in this context.
In conclusion, the results of current studies of serum IFX concentrations in patients with SA are not conclusive. It would be necessary to carry out an adequate study to definitively conclude whether TDM could help in making therapeutic decisions in this disease, as occurs in other immune mediated inflammatory diseases.
5.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY.52
COMPETING INTERESTS
There are no competing interests to declare.
Fobelo Lozano MJ, Serrano Giménez R, Sánchez Fidalgo S. Therapeutic drug monitoring of infliximab in spondyloarthritis. A review of the literature. Br J Clin Pharmacol. 2019; 85: 2264–2279. 10.1111/bcp.14062
REFERENCES
- 1. Sfikakis PP. The first decade of biologic TNF antagonists in clinical practice: lessons learned unresolved issues and future directions. Curr Dir Autoimmun. 2010;11:180‐210. [DOI] [PubMed] [Google Scholar]
- 2. Moots RJ, Xavier RM, Mok CC, et al. The impact of anti‐drug antibodies on drug concentrations and clinical outcomes in rheumatoid arthritis patients treated with adalimumab, etanercept, or infliximab: results from a multinational, real‐world clinical practice, non‐interventional study. PLoS ONE. 2017;12(4):e0175207 10.1371/journal.pone.0175207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thomas SS, Borazan N, Barroso N, et al. Comparative immunogenicity of TNF inhibitors: impact on clinical efficacy and tolerability in the Management of Autoimmune Diseases. A Systematic Review and Meta‐Analysis BioDrugs. 2015;29(4):241‐258. 10.1007/s40259-015-0134-5 [DOI] [PubMed] [Google Scholar]
- 4. Pascual‐Salcedo D, Plasencia C, Ramiro S, et al. Influence of immunogenicity on the efficacy of long‐term treatment with infliximab in rheumatoid arthritis. Rheumatology (Oxford). 2011;50(8):1445‐1452. 10.1093/rheumatology/ker124 [DOI] [PubMed] [Google Scholar]
- 5. iatdmct.org [Internet]. Rochester (NY); IATDMCT; [Cited May 22 2019]. Available from: https://www.iatdmct.org/about‐us/about‐association/about‐definitions‐tdm‐ct.html.
- 6. Laine J, Jokiranta TS, Eklund KK, Väkeväinen M, Puolakka K. Cost‐effectiveness of routine measuring of serum drug concentrations and anti‐drug antibodies in treatment of rheumatoid arthritis patients with TNF‐α blockers. Biol Theory. 2016;10:67‐73. 10.2147/BTT.S96982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harzallah I, Rigaill J, Williet N, Paul S, Roblin X. Golimumab pharmacokinetics in ulcerative colitis: a literature review. Therap Adv Gastroenterol. 2017;10(1):89‐100. 10.1177/1756283X16676194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martelli L, Olivera P, Roblin X, Attar A, Peyrin‐Biroulet L. Cost‐effectiveness of drug monitoring of anti‐TNF therapy in inflammatory bowel disease and rheumatoid arthritis: a systematic review. J Gastroenterol. 2017;52(1):19‐25. 10.1007/s00535-016-1266-1 [DOI] [PubMed] [Google Scholar]
- 9. Passot C, Mulleman D, Bejan‐Angoulvant T, et al. The underlying inflammatory chronic disease influences infliximab pharmacokinetics. MAbs. 2016;8(7):1407‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takeuchi T, Miyasaka N, Inoue K, Abe T, Koike T. Impact of trough serum level on radiographic and clinical response to infliximab plus methotrexate in patients with rheumatoid arthritis: results from the RISING study. Mod Rheumatol. 2009;19(5):478‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pouw MF, Krieckaert CL, Nurmohamed MT, et al. Key findings towards optimising adalimumab treatment: the concentration‐effect curve. Ann Rheum Dis. 2015;74(3):513‐518. [DOI] [PubMed] [Google Scholar]
- 12. Van Herwaarden N, Van Den Bemt BJF, Wientjes MHM, Kramers C, Den Broeder AA. Clinical utility of therapeutic drug monitoring in biological disease modifying anti‐rheumatic drug treatment of rheumatic disorders: a systematic narrative review. Expert Opin Drug Metab Toxicol. 2017. Aug;13(8):843‐857. 10.1080/17425255.2017.1353602 [DOI] [PubMed] [Google Scholar]
- 13. Krzysiek R, Breban M, Ravaud P, et al. Circulating concentration of infliximab and response to treatment in ankylosing spondylitis: results from a randomized control study. Arthritis Rheum. 2009;61(5):569‐576. 10.1002/art.24275 [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi S, Yoshinari T. A multicenter, open‐label, long‐term study of three‐year infliximab administration in Japanese patients with ankylosing spondylitis. Mod Rheumatol. 2017;27(1):142‐149. 10.1080/14397595.2016.1176635 [DOI] [PubMed] [Google Scholar]
- 15. Hernández‐Flórez D, Valor L, de la Torre I, et al. Comparison of two ELISA versions for infliximab serum levels in patients diagnosed with ankylosing spondylitis. Rheumatol Int. 2015;35(6):1021‐1025. 10.1007/s00296-014-3180-2 [DOI] [PubMed] [Google Scholar]
- 16. Park W, Hrycaj P, Jeka S, et al. A randomised, double‐blind, multicentre, parallel‐group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT‐P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72(10):1605‐1612. 10.1136/annrheumdis-2012-203091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Méric JC, Mulleman D, Ducourau E, et al. Therapeutic drug monitoring of infliximab in spondyloarthritis: an observational open‐label study. Ther Drug Monit. 2011;33(4):411‐416. 10.1097/FTD.0b013e318224f83d [DOI] [PubMed] [Google Scholar]
- 18. Chu Miow Lin D, Mulleman D, Azzopardi N, et al. Trough infliximab concentration may predict long‐term maintenance of infliximab in ankylosing spondylitis. Scand J Rheumatol. 2010;39(1):97‐98. 10.3109/03009740903177745 [DOI] [PubMed] [Google Scholar]
- 19. Ducourau E, Mulleman D, Paintaud G, et al. Antibodies toward infliximab are associated with low infliximab concentration at treatment initiation and poor infliximab maintenance in rheumatic diseases. Arthritis Res Ther. 2011;13(3):R105 10.1186/ar3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Vries MK, Wolbink GJ, Stapel SO, et al. Decreased clinical response to infliximab in ankylosing spondylitis is correlated with anti‐infliximab formation. Ann Rheum Dis. 2007;66(9):1252‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Inciarte‐Mundo J, Ramirez J, Hernández MV, et al. Calprotectin and TNF trough serum levels identify power Doppler ultrasound synovitis in rheumatoid arthritis and psoriatic arthritis patients in remission or with low disease activity. Arthritis Res Ther. 2016;18(1):160 10.1186/s13075-016-1032-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Almirall M, Gimeno R, Salman‐Monte TC, Iniesta S, Lisbona MP, Maymó J. Drug levels, immunogenicity and assessment of active sacroiliitis in patients with axial spondyloarthritis under biologic tapering strategy. Rheumatol Int. 2016;36(4):575‐578. 10.1007/s00296-016-3428-0 [DOI] [PubMed] [Google Scholar]
- 23. Arstikyte I, Kapleryte G, Butrimiene I, Venalis A. Influence of immunogenicity on the efficacy of long‐term treatment with TNF α blockers in rheumatoid arthritis and Spondyloarthritis patients. Biomed Res Int. 2015;2015:604872‐604810. 10.1155/2015/604872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Plasencia C, Pascual‐Salcedo D, Nuño L, et al. Influence of immunogenicity on the efficacy of long‐term treatment of spondyloarthritis with infliximab. Ann Rheum Dis. 2012;71(12):1955‐1960. 10.1136/annrheumdis-2011-200828 [DOI] [PubMed] [Google Scholar]
- 25. Mulleman D, Chu Miow Lin D, Ducourau E, Emond P, Ternant D, Magdelaine‐Beuzelin C Valat JP, Paintaud G, Goupille P. Trough infliximab concentrations predict efficacy and sustained control of disease activity in rheumatoid arthritis. Ther Drug Monit 2010; 32:232–236. 10.1097/FTD.0b013e3181cc6fef. [DOI] [PubMed] [Google Scholar]
- 26. Wolbink GJ, Voskuyl AE, Lems WF, de Groot E, Nurmohamed MT, Tak PP Dijkmans BA, Aarden L. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis 2005; 64:704–707. 10.1136/ard.2004.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C Li S, Dooley LT, Griffiths CE, EXPRESS Study Investigators . Infliximab induction and maintenance therapy for moderate‐to‐severe psoriasis: a phase III, multicentre, double‐blind trial. Lancet (London, England) 2005; 366:1367–1374. 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- 28. Van den Bemt BJ, den Broeder AA, Wolbink GJ, et al. The combined use of disease activity and infliximab serum trough concentrations for early prediction of (non‐)response to infliximab in rheumatoid arthritis. Br J Clin Pharmacol. 2013;76(6):939‐945. 10.1111/bcp.12142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kiely PD. Biologic efficacy optimization‐‐a step towards personalized medicine. Rheumatology (Oxford). 2016;55(5):780‐788. 10.1093/rheumatology/kev356 [DOI] [PubMed] [Google Scholar]
- 30. Brandse JF, Mould D, Smeekes O, et al. A real‐life population pharmacokinetic study reveals factors associated with clearance and immunogenicity of infliximab in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(4):650‐660. [DOI] [PubMed] [Google Scholar]
- 31. Hayashi S, Suzuki K, Yoshimoto K, et al. Early prognostic factors associated with the efficacy of infliximab treatment for patients with rheumatoid arthritis with inadequate response to methotrexate. Rheumatol Ther. 2016;3(1):155‐166. 10.1007/s40744-015-0022-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti‐TNF therapy in Crohn's disease ‐ algorithm for practical management. Aliment Pharmacol Ther. 2016;43(1):30‐51. 10.1111/apt.13445 [DOI] [PubMed] [Google Scholar]
- 33. Mould DR. The pharmacokinetics of biologics: a primer. Dig Dis. 2015;33(Suppl 1):61‐69. [DOI] [PubMed] [Google Scholar]
- 34. Valor L, Hernández‐Flórez D, de la Torre I, et al. Investigating the link between disease activity and infliximab serum levels in rheumatoid arthritis patients. Clin Exp Rheumatol. 2015;33(6):805‐811. [PubMed] [Google Scholar]
- 35. Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology. 2014;147(6):1296‐1307. 10.1053/j.gastro.2014.08.035 [DOI] [PubMed] [Google Scholar]
- 36. Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DC, Sturrock RD. Ankylosing spondylitis and HL‐A 27. Lancet. 1973;301(7809):904‐907. [DOI] [PubMed] [Google Scholar]
- 37. Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(10):633‐659. 10.2165/11535960-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 38. Ternant D, Bejan‐Angoulvant T, Passot C, Mulleman D, Paintaud G. Clinical pharmacokinetics and pharmacodynamics of monoclonal antibodies approved to treat rheumatoid arthritis. Clin Pharmacokinet. 2015;54(11):1107‐1123. 10.1007/s40262-015-0296-9 [DOI] [PubMed] [Google Scholar]
- 39. Bar‐Yoseph H, Levhar N, Selinger L, et al. Early drug and anti‐infliximab antibody levels for prediction of primary nonresponse to infliximab therapy. Aliment Pharmacol Ther. 2018;47(2):212‐218. 10.1111/apt.14410 [DOI] [PubMed] [Google Scholar]
- 40. Hendy P, Hart A, Irving P. Anti‐TNF drug and antidrug antibody level monitoring in IBD: a practical guide. Frontline Gastroenterol. 2016;7(2):122‐128. 10.1136/flgastro-2014-100527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mok CC, van der Kleij D, Wolbink GJ. Drug levels, anti‐drug antibodies, and clinical efficacy of the anti‐TNFα biologics in rheumatic diseases. Clin Rheumatol. 2013;32(10):1429‐1435. [DOI] [PubMed] [Google Scholar]
- 42. Bendtzen K, Geborek P, Svenson M, Larsson L, Kapetanovic MC, Saxne T. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum. 2006;54(12):3782‐3789. [DOI] [PubMed] [Google Scholar]
- 43. Rosas J, Llinares‐Tello F, Senabre‐Gallego JM, et al. Obesity decreases clinical efficacy and levels of adalimumab in patients with ankylosing spondylitis. Clin Exp Rheumatol. 2017;35(1):145‐148. [PubMed] [Google Scholar]
- 44. Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti‐tumor necrosis factor alpha monoclonal antibody combined with low‐dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41(9):1552‐1563. [DOI] [PubMed] [Google Scholar]
- 45. Martín‐López M, Carmona L, Balsa A, et al. Serum drug levels of biologic agents in the management of rheumatoid arthritis and spondyloarthritis: a systematic review. Rheumatol Int. 2018;38(6):975‐983. 10.1007/s00296-018-4022-4 [DOI] [PubMed] [Google Scholar]
- 46. Ternant D, Mulleman D, Lauféron F, et al. Influence of methotrexate on infliximab pharmacokinetics and pharmacodynamics in ankylosing spondylitis. Br J Clin Pharmacol. 2012;73(1):55‐65. 10.1111/j.1365-2125.2011.04050.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jani M, Barton A, Warren RB, Griffiths CE, Chinoy H. The role of DMARDs in reducing the immunogenicity of TNF inhibitors in chronic inflammatory diseases. Rheumatology (Oxford). 2014;53(2):213‐222. 10.1093/rheumatology/ket260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Strand V, Balsa A, Al‐Saleh J, et al. Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. BioDrugs. 2017;31(4):299‐316. 10.1007/s40259-017-0231-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bornstein G, Lidar M, Langevitz P, Fardman A, Ben‐Zvi I, Grossman C. The prevalence and clinical effect of immunogenicity of TNF‐α blockers in patients with axial spondyloarthritis. Clin Exp Rheumatol. 2018. Mar‐Apr;36(2):228‐232. [PubMed] [Google Scholar]
- 50. Plasencia C, Pascual‐Salcedo D, García‐Carazo S, et al. The immunogenicity to the first anti‐TNF therapy determines the outcome of switching to a second anti‐TNF therapy in spondyloarthritis patients. Arthritis Res Ther. 2013. Jul 26;15(4):R79 10.1186/ar4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Benucci M, Gobbi FL, Bandinelli F, et al. Safety, efficacy and immunogenicity of switching from innovator to biosimilar infliximab in patients with spondyloarthritis: a 6‐month real‐life observational study. Immunol Res. 2017;65(1):419‐422. 10.1007/s12026-016-8843-5 [DOI] [PubMed] [Google Scholar]
- 52. Harding SD, Sharman JL, Faccenda E, et al. he IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res. 2018;46:D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]


