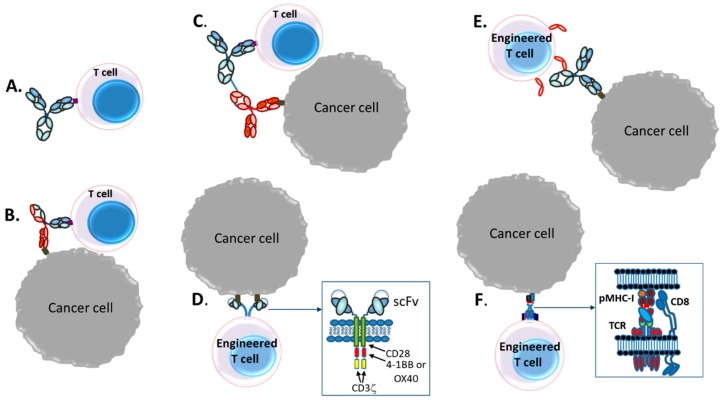
Figure 1.
Examples of T-cell based therapeutics in clinical development. (A) Inhibition of checkpoint receptors such as PD-1 and CTLA-4 to improve T-cell activity [18]; (B) T-cell redirection with bispecific antibodies (TRBAs) in which one binding arm recognizes a tumor antigen and the other binding arm recognizes CD3ε on T-cells [17,19,20]; (C) Autologous T-cells activated ex vivo, combined with bispecific antibody conjugates recognizing tumor antigen with one mAb and CD3ε on T cells with the other mAb, followed by re-administration to the patient to kill tumors [21]; (D) Genetically engineered autologous chimeric antigen receptor (CAR)-T cells in which an antibody, typically a single chain variable fragment (scFv), fused to intracellular T-cell activation domains such as CD28, 4-1BB, OX40 and CD3ζ, replace the function of the T-cell receptor (TCR), making the T-cells killers of specific antigen-bearing cells [22,23,24]; (E) Autologous or allogeneic T-cells or NK cells genetically engineered with FcγRIIIa (CD16a), which, when administered with an anti-tumor monoclonal antibody (mAb) such as the anti-CD20 mAb, rituximab, binds to the Fc of the antibodies and functionally redirects the T-or NK-cells to the tumor to kill the cancer cells [25]; (F). Autologous T cells with engineered TCRs.