Abstract
Sexual differences in morphology can evolve by sexual selection and/or natural selection. In some species, only males have morphological structures that are used as weapons. Since some weapons may also be used for defensive purposes, males and females may behave differently towards predators. In some species of harvestmen (Arachnida and Opiliones), males have sharp apophyses (“spines”) on their 4th pair of legs whereas females lack them. Those apophyses are used in male–male fights and in antipredatory behaviors. The harvestmen antipredatory repertory also encompasses passive defenses such as thanatosis (death feigning), retaliation (attack on predators), and chemical defense. Due to the sexual differences on weaponry, we hypothesized that males and females of Mischonyx cuspidatus (Gonyleptidae) rely on different defensive strategies. We experimentally induced males and females to perform 3 defensive behaviors: thanatosis, pinching with legs, and chemical release. We predicted that females would engage more in passive and chemical defenses than males, whereas males would rely more on retaliation than females. As expected, females performed thanatosis more often than males. Likewise, males performed retaliation more often than females. We did not find differences in the rate of chemical defense use between the sexes. This study provides evidence that due to sexual dimorphism, alternative antipredatory behaviors may have been selected in the different sexes in M. cuspidatus.
Keywords: Arachnida, death feigning, nipping, thanatosis, weapon, Opiliones
Sexual and natural selection may give rise to intersexual differences in size, color, and shape (Darwin 1859; Andersson 1994). Sexual dimorphism can evolve both through mate choice and male–male contest competition. In the first case, female preference may select for specific male traits, favoring the evolution of ornaments. Regarding male–male contests, advantageous traits in competition such as weapons would be selected (McCullough et al. 2016). The dimorphism in weaponry is widespread among animals. In vertebrates, mammals are a typical example of sexual dimorphism with the presence of antlers in males of cervids, horns in males of bovids, and tusks in Asiatic elephants (Emlen 2008, 2014). Concerning invertebrates, males of stag, and rhinoceros beetles (Lucanidae and Scarabaeidae, respectively) have enlarged mandibles and horns, and males of leaf-footed bugs (Coreidae) and “Nutcracker” Camel Crickets (Rhaphidophoridae) have enlarged hind legs bearing spines that are lacking in females (Miyatake 1997; reviewed in Emlen 2008, 2014; Conroy and Gray 2015).
Natural selection acts on both morphology and performances, that is, the functional capacities and behavior (Irschick et al. 2008). Consequently, in sexually dimorphic species different behavioral responses could be selected for each sex. As a result, males and females may behave differently when facing challenges that somehow relate to their dimorphic morphology. The defensive context is a fructiferous field for studying sexual differences in behavior and its relations with sexual dimorphism in morphology, because prey is under strong selection since an inappropriate behavior when facing a predator may result in death (Dawkins and Krebs 1979). Indeed, the literature shows examples of the relation between sexual dimorphism and different defensive strategies. In some sexually dimorphic turtles, with females larger and heavier than males, the time to emerge from their shells after being overturned is greater for females than males. The authors suggest that this might be due to an increased risk of being detected by predators because of female’s size (Ibáñez et al. 2014). In stalk-eyed flies, males have greater eye span than females and exhibit more aggressive displays against a spider (a potential predator) than females. The authors have suggested that this is because of poorer flight capability of males. Thus, the aggressiveness would compensate this handicap (Worthington and Swallow 2010). In a species of scorpion in which males tend to be thinner and have a greater sprint ability than females, females exhibited shorter latencies to sting and stung more frequently than males when experimentally threatened (Miller et al. 2016).
In arachnids of the order Opiliones, the most studied defensive strategy of harvestmen is their chemical defense, which consists of the emission of secretions through gland openings usually located dorso-laterally on the prosoma (Hara et al. 2005; Gnaspini and Hara 2007; Machado and Pomini 2008; Raspotnig et al. 2014). Laboratory experiments show that defensive chemicals per se, when experimentally applied to palatable food items are effective against some species of spiders, ants, and frogs (Machado et al. 2005). However, surprisingly, it is not used or is inefficient in tests that paired actual harvestmen with predators, such as spiders and scorpions (Eisner et al. 2004; Segovia et al. 2015a, 2015b; Albín and Toscano-Gadea 2015). The defensive strategies of harvestmen also includes, a hard integument, fleeing, stridulating, performing intense leg tapping, showing appendotomy, thanatosis, and retaliation, such as pinching with pedipalps and chelicerae as well as pinching with legs IV that bear sharp apophyses in some species (hereafter called nipping) (Gnaspini and Hara 2007; Pomini et al. 2010; Souza and Willemart 2011; Dias and Willemart 2013; Dias et al. 2014; Albín and Toscano-Gadea 2015; Segalerba and Toscano-Gadea 2016). Nipping behavior is widespread in the family Gonyleptidae (Caetano and Machado 2013) and, besides the antipredatory context, it is known to be used in male–male fights (Nazareth and Machado 2009; Willemart et al. 2009) and in nest defense (Machado et al. 2004; Nazareth and Machado 2009).
Sexual dimorphism has been shown within the 4 extant suborders of Opiliones (Pinto-da-Rocha and Giribet 2007). Representatives of the family Gonyleptidae (Suborder Laniatores) may have sexually dimorphic legs IV, with males typically having thicker femurs with apophyses that are lacking in females (Pinto-da-Rocha and Giribet 2007). Because of these intersexual morphological differences, some degree of sexually dimorphic behavior is expected, especially with regard to antipredatory strategies. Males of the harvestman Mischonyx cuspidatus (Roewer, 1913) bear sharp apophyses on legs IV that are used in nipping behavior (Segovia et al. 2015a), whereas females’ legs lack such weapons. Moreover, they seem to be larger and have thicker femurs, which would allow more space for muscles important for delivering more powerful nippings. Thus, males and females could behave differently when facing a predator. Here, in order to explicitly test the sexual dimorphism on body shape, we measured body parameters of the harvestmen and compared allometric patterns between the sexes. Then, taking into account the lack of weapons on IV legs of females, we experimentally tested the hypothesis that males and females rely on different antipredatory strategies. We expected that males would rely more on nipping than females. In addition to nipping, we specifically looked at passive and chemical defenses. We expected that females would perform thanatosis and release defensive chemical more often than males.
Materials and Methods
Study species and laboratory conditions
We collected the individuals of M. cuspidatus under trunks at the Parque Ecológico do Tietê, São Paulo city, São Paulo State, Brazil (23°25S, 46°28’W) in September 2015. We chose M. cuspidatus because this species is sexually dimorphic (Figure 1), individuals engage in thanatosis (Pereira et al. 2004) and use both chemical and mechanical defenses (Hara et al. 2005; Segovia et al. 2015a). Thus, M. cuspidatus is an ideal model for studying sexual differences on defensive strategies.
Figure 1.

Differences on weaponry between sexes of the harvestman Mischonyx cuspidatus. Males bearing sharp apophysis (left side) and unarmed females (right side).
We kept the harvestmen individually in plastic boxes (∼13.5 cm; 9.5cm; Height 4.5 cm) with paper towel covering the substrate. We fed all the collected harvestmen with dog food (Pedigree Vital Pro®) and conducted the tests later in the same week (between 1 day and 5 days after they were fed). A wet cotton ball inside a plastic bottle cap provided water for the animals. We maintained the animals under a natural dark cycle (∼12: 12). The experiments of antipredatory strategies were run in September of 2015. After the end of the experiments, we fixed the animals in 70% ethanol in order to take morphological measurements. We first tested the individuals for passive behaviors, then for mechanical and chemical defenses. Nonetheless, for the sake of clarity, we first present the morphological then the behavioral data. The same experimenter held the animals in every trial of both experiments to minimize biases.
Morphological and allometric differences
We conducted this step to investigate allometric differences between males and females of M. cuspidatus. In other words, we aimed to show to what degree males and females differ in morphology. We measured the body size of each harvestman (66 males and 64 females) using a Leica M205 C stereomicroscope and the software Leica Application Suite V3.7. We used dorsal scute length as standardized measure of size (see Willemart et al. 2009; Buzatto et al. 2014). The measures of dorsal scute width and diameter of femur IV were considered as potential parameters of allometric sexual dimorphism (Willemart et al. 2009). We took the leg measures from the right legs, except in 2 cases in which we measured the left leg because the right one was absent.
Passive defenses
In this experiment, we tested if females engage more in thanatosis than males. We performed the following procedures to induce thanatosis: first, we opened the plastic box where we maintained the harvestman, and then we gently held the harvestman by the femur of its 4th left leg and dropped it from a distance of ∼20 cm from a substrate of filter paper. We defined thanatosis as a posture in which the harvestman stayed rigid with the legs retracted close to the body with no more than 1 leg extended (modified from Gnaspini and Hara 2007). We considered the end of thanatosis when the individual extended all legs. If the harvestman stayed stationary with extended legs for more than 3 s before resuming movement, we considered that it performed freezing behavior (see Chelini et al. 2009). This definition was used to aggregate all different postures adopted by harvestmen whereas standing still except thanatosis as we have defined. The harvestmen were tested between ∼1:00 PM and 6:00 PM, a period in which M. cuspidatus are usually resting (Pereira et al. 2004) and can potentially be located in their refuges by predators. We tested males and females alternately to avoid potential biases.
Mechanical and chemical defenses
Here we tested if males rely more on nipping than females, and if females rely more on chemical defenses than males. We held the harvestman dorso-ventrally between the thumb and index fingers at the region of the opisthosoma for ∼10 s in order to induce nipping behavior and the release of defensive chemicals (Hara et al. 2005; Segovia et al. 2015c). Only one experimenter manipulated the animals in order to minimize differences on the strength applied and care has been taken not to bias the results. All individuals were tested only once in the same day between ∼10:00 PM and 3:00 AM. We alternate males and females in this experiment to avoid biases.
Statistical analysis
To test for allometric differences, we ran t tests using sex as an independent variable. The dependent variables were (i) dorsal scute width divided by dorsal scute length and (ii) diameter of femur IV divided by dorsal scute length. These divisions were performed to provide single variables accounting for allometry, allowing for sexual comparisons. For the data regarding defensive strategy, we performed chi-square tests comparing the frequency of thanatosis, freezing, and chemical defense between males and females. We compared the time spent in thanatosis and freezing between the sexes with a Mann–Whitney test. All tests were performed in the software Systat 12.
Results
Allometric differences
The ratio between the dorsal scute width and dorsal scute length of M. cuspidatus (mean ± SD) was greater for males (1.01 ± 0.07; N = 59; MiN = 0.89; Max = 1.13) than for females (0.96 ± 0.08; N = 53; MiN = 0.79; Max = 1.13) (t = 3.986; df = 110; P < 0.001; Figure 2A). Similarly, the ratio between the diameter of the femur IV and the dorsal scute length was greater for males (0.13 ± 0.02; N = 59; MiN = 0.09; Max = 0.18) than for females (0.11 ± 0.01; N = 53; MiN = 0.08; Max = 0.13) (t = 8.354; df = 110; P < 0.001; Figure 2B).
Figure 2.
Allometric differences between sexes in the harvestman Mischonyx cuspidatus. (A) Dorsal scute width plotted against dorsal scute length; (B) Diameter of femur IV plotted against dorsal scute length. (C) Femur III length plotted against dorsal scute length. Black diamonds represent males, empty circles represent females.
Passive defenses
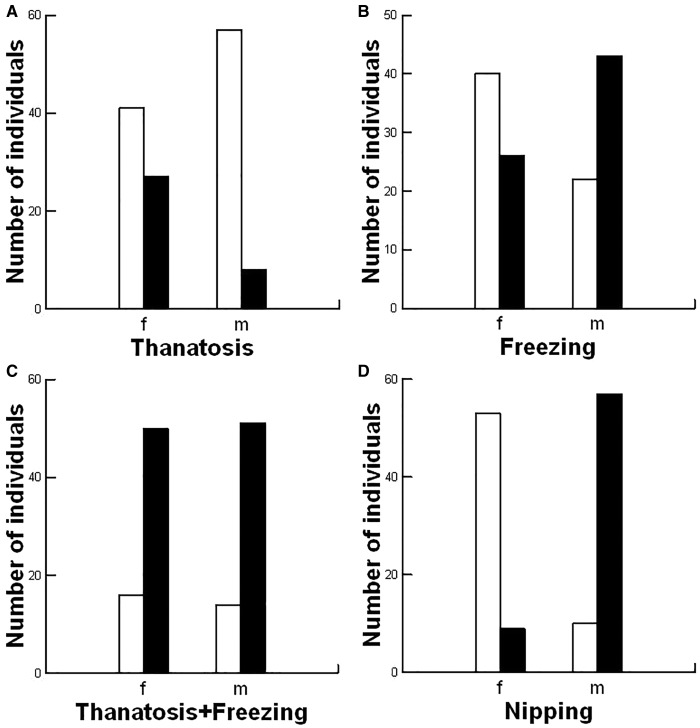
The number of individuals that performed thanatosis was different between males (8 out of 65) and females (27 out of 66) (χ2 = 13.682; df = 1; P < 0.001; Figure 3A). Similarly, the number of individuals that performed freezing behavior was different between males (42 out of 65) and females (26 out of 66) (χ 2 = 8.345; df = 1; P = 0.004; Figure 3B). However, the number of individuals that exhibited passive defenses (thanatosis+freezing polled together) was not different between the sexes (50 females out of 66 and 51 males out of 65; χ2 = 0.136; df = 1; P = 0.713; Figure 3C).
Figure 3.
Between sex comparisons of rates for defensive behaviors of the harvestman Mischonyx cuspidatus. (A) Thanatosis; (B) Freezing; (C) Thanatosis + freezing pooled together; (D) Nipping. Black bars: the number of individuals performing a behavior. White bars: number of individuals that did not perform the behavior.
The time spent in thanatosis (in seconds) was not different (Mann–Whitney U test: U = 103.5; Nfemales = 27, Nmales = 8; P = 0.860) between females (MediaN = 108; MiN = 12; Max = 1200) and males (MediaN = 135; MiN = 31; Max = 633). Likewise, the time spent in freezing (in seconds) was not different (Mann–Whitney U test: U = 565.5; Nfemales = 26, Nmales = 43; P = 0.936) between females (MediaN = 53.5; Minimum = 4; Maximum = 1504) and males (MediaN = 36; MiN = 5; Max = 399). We did not observe any harvestmen releasing defensive chemicals when manipulated in this experiment.
Mechanical and chemical defenses
The number of individuals that performed nipping was different between the males (57 out of 67) and females (9 out of 62) (χ2 = 64.161; df = 1; P < 0.001; Figure 3D). The number of individuals that released defensive chemicals was not different between the sexes (χ2 = 0.016; df = 1; P = 0.901). Twenty-one (out of 61) females and 22 (out of 67) males released defensive chemicals.
Discussion
In this work, we investigated the presence of sexual differences in antipredatory behavior of M. cuspidatus, considering that males have weaponry that females do not. Our morphological results clearly show a sexual dimorphism in body shape (carapace and femur width) of M. cuspidatus. With regard to the defensive strategies, we found that more females performed thanatosis than males. On the contrary, more males performed freezing than females. Furthermore, when thanatosis and freezing were pooled together, males and females performed passive defenses similarly. The time spent both in thanatosis and freezing was not different between the sexes. “Sexual differences in defensive behavior have also been found in beetles. Males spend less time in thanatosis at night than during the day, whereas in females there were no differences. (Miyatake 2001a). In addition, females needed more days of starvation to show a decrease in proportion of individuals performing death feigning (Miyatake 2001b).” With respect to mechanical defenses, males exhibited nipping more often than females. We failed to find sexual differences in the number of individuals releasing the defensive chemical.
We found that, proportionally, males have larger bodies and larger femur of the 4th leg than females. Similarly, previous studies have reported allometric differences between the sexes in the family Gonyleptidae (Willemart et al. 2009; Buzatto et al. 2014). These allometric sexual differences can be the result of selective pressures involving male-male fights, which are common at least in species of Gonyleptidae (Willemart et al. 2009; Zatz et al. 2011; Buzatto et al. 2014). Females performed thanatosis more often than males. Little is known about the effectiveness of thanatosis as antipredatory behavior in harvestman (but see Segalerba and Toscano-Gadea 2016). However, several studies with arthropods provide evidence of thanatosis’ efficacy against predators. A beetle strain selected to have a longer thanatosis’ time survived more when facing a predatory spider than the strain selected for a short latency of thanatosis (Miyatake et al. 2004). Thanatosis effectively prevented phasmids from being preyed upon by mantids (Reitze and Nentwig 1991). More active individuals of damselflies were preyed upon by fish and dragonflies earlier than frozen individuals mimicking thanatosis (Gyssels and Stoks 2005). In addition, the tonically immobilized selfish hypothesis proposes that individuals performing thanatosis improve their own survivorship by sacrificing their neighbors in group living species (Miyatake et al. 2009). M. cuspidatus is often found in aggregations. Thus, avoiding being caught by feigning death whereas others do not (i.e., the tonically immobilized selfish hypothesis) (Miyatake et al. 2009) could also apply to this harvestman species.
Males performed freezing behavior more often than females. However, when pooled together (thanatosis + freezing) the frequency of passive defenses was not different between males and females. Why do males and females adopt different postures for standing still? Assuming that visual predators learn search images of their prey (Shettleworth 2010), the posture of thanatosis could confuse the predator by distorting the typical body shape of the prey. So, if the posture of thanatosis is advantageous males should perform this behavior as often as females, but they do not. A possible explanation for this difference on frequency of thanatosis is that, since males might not be able to use nipping during thanatosis (because the legs are already flexed), they would perform freezing (legs extended) more often. Females, on the contrary, do not use nipping as often as males do, and the females’ nipping is potentially less effective than the nipping of males, thus, it seems reasonable that females perform a putative cryptic posture more frequently. Males and females did not differ in the time they took displaying both thanatosis and freezing. Although immobility can be efficient against predators (discussed above), this behavior comes with trade-offs. A previous study has shown that freezing resulted in less food consumption and less weight gain in a harvestman species (Chelini et al. 2009). The ideal time spent in passive defenses must counter-balance the time to avoid predation with the physiological needs, such as feeding and other important activities, for example reproduction (see Miyatake 2001a). In the case of harvestmen, seeking for refuges to avoid desiccation is also important, since water loss is critical for this group (see review in Santos 2007). Hence, the fact that males and females stand still for a similar time may suggest that the optima are similar for both sexes.
The nipping behavior was performed more often by males than females. Males bear sharp apophyses on their 4th femur, but females do not. We therefore expect the nipping behavior to be more effective in males since the apophyses can harm predators (see Segovia et al. 2015a). Nonetheless, females also perform nipping (Gnaspini and Hara 2007; this study). Nipping has been observed against natural predators such as spiders and scorpions (Segovia et al. 2015a; Albín and Toscano-Gadea 2015; Segalerba and Toscano-Gadea 2016). However, in only one case it was reported that a predatory ctenid spider, that was retaliated by nipping and moved away. The legs IV of the harvestman only touched the legs of the ctenid spider, which quickly retreated unharmed (Dias and Willemart 2013). In this case, the nipping may, therefore, be considered to be a deimatic behavior, that is, a behavior that usually is not harmful per se, but can frighten the predator (Edmunds 1974). If predators often retreat in such cases, the nipping could also be positively selected in females. However, due to differences in morphology it is possible that males can perform a stronger and more dangerous nipping than females. Thus, it could contribute to the differences on the frequency of nipping between the sexes. It is worthwhile mentioning that nipping behavior is also used in male–male fights (Willemart et al. 2009), which could also contribute to the differential frequency of nipping among the sexes.
Another important finding was that none of the individuals of M. cuspidatus released defensive chemicals in the first experiment, when we held them by one of their legs. However, when held by the cephalothorax in the second experiment, several individuals did release chemicals. Considering that the latter situation is probably interpreted as more dangerous than the former, these different approaches might represent different levels of threat. Therefore, our results are in agreement with the threat sensitive hypothesis which postulates that the animals assess and respond differentially to different levels of threat (Helfman 1989; see also Segovia et al. 2015a). Since harvestmen have several lines of defenses (Pomini et al. 2010) and chemical defense is costly (Nazareth and Machado 2015), it is expected that it will be used only in high levels of threat. Under this perspective, the absence of differences on the frequency of chemical defensive releasing among the sexes seems to be reasonable. At high levels of threat, the animals are expected to invest their best in defense. Similar results with harvestman show no differences in the defensive behavior between sexes (Segovia et al. 2015c).
As far as we know, this is the first time that sexual differences on antipredatory behavior are demonstrated experimentally among harvestmen. Nonetheless, many questions remain open. A comparative approach testing the hypothesis that differences on defenses are associated with differences on weaponry can be rewarding. This question can be investigated by comparing the frequency of thanatosis and nipping within and between species with and without sexual dimorphism on weaponry. Another approach to shed light on the explanations for intersexual differences in defense behavior is testing the adaptive value of different strategies (nipping, thanatosis, and freezing). For doing so, it is important to test the efficacy of defense against natural predators as stressed by Segovia et al. (2015b). At this point, except for a few arachnids that elicited nipping on harvestmen, little is known about which predators evoke each of the harvestmen’s different defensive behaviors. Thus, naturalistic studies are still required.
As expected, we found behavioral differences between sexes in M. cuspidatus. These results are in agreement with the idea that sexually dimorphic traits may be influencing which antipredatory behavior is more advantageous in each sex. These findings are quite compelling because sexual dimorphism may have evolved initially in a sexual context, and subsequently affected the evolution of sexual differences in antipredatory behavior. More evidence showing associations between sexual dimorphism and differences in antipredatory behavior is still desirable. To this end, a comparison of the survivorship of individuals that exhibit the typical behavior of the opposite sex, with their counterparts who exhibit the most common behavior of their own sex, may be of interest. For example, in M. cuspidatus, females perform thanatosis more often than males. However, some males also perform thanatosis. So, it may be rewarding to look for males that perform thanatosis and compare their survivorship with males who behave typically (do not perform thanatosis). Finally, we hope this study will increase interest in the sexual differences in defensive behavior of species with dimorphic weaponry.
Acknowledgments
The authors would like to thank Rafaela Molina for helping them to collect the animals. The authors also thank Guilherme Gainett, Laura Senteno, Rafael Moura, and 3 anonymous reviewers that greatly helped us to improve this manuscript.
Funding
Funding was provided by CAPES to J.M.G.S and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) process 2014/19191-3 to J.M.G.S and 2015/01815-9 to R.H.W.
Authors’ Contributions
J.M.G.S, G.P.M, and R.H.W. designed the experiments; J.M.G.S and G.P.M. collected the data; J.M.G.S. and G.P.M. analyzed the videos; J.M.G.S conducted the statistical tests; J.M.G.S. wrote the manuscript; G.P.M and R.H.W revised and gave suggestions on the manuscript.
References
- Albín A, Toscano-Gadea CA, 2015. Predation among armored arachnids: bothriurus bonariensis (Scorpions, Bothriuridae) versus four species of harvestmen (Harvestmen, Gonyleptidae). Behav Process 121:1–7. [DOI] [PubMed] [Google Scholar]
- Andersson MB, 1994. Sexual Selection. Princeton (NJ: ): Princeton University Press. [Google Scholar]
- Buzatto BA, Tomkins JL, Simmons LW, Machado G, 2014. Correlated evolution of sexual dimorphism and male dimorphism in a clade of neotropical harvestmen. Evolution 68:1671–1686. [DOI] [PubMed] [Google Scholar]
- Caetano DS, Machado G, 2013. The ecological tale of Gonyleptidae (Arachnida, Opiliones) evolution: phylogeny of a Neotropical lineage of armoured harvestmen using ecological, behavioural and chemical characters. Cladistics 29:589–609. [DOI] [PubMed] [Google Scholar]
- Chelini MC, Willemart RH, Hebets EA, 2009. Costs and benefits of freezing behaviour in the harvestman Eumesosoma roeweri (Arachnida, Opiliones). Behav Process 82:153–159. [DOI] [PubMed] [Google Scholar]
- Conroy LP, Gray DA, 2015. Male armaments and reproductive behavior in “nutcracker” camel crickets (Rhaphidophoridae, Pristoceuthophilus). Insects 6:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C, 1859. On the Origin of Species. London: Murray. [Google Scholar]
- Dawkins R, Krebs JR, 1979. Arms races between and within species. Proc R Soc Lond B 205:489–511. [DOI] [PubMed] [Google Scholar]
- Dias BC, Willemart RH, 2013. The effectiveness of post–contact defenses in a prey with no pre-contact detection. Zoology 116:168–174. [DOI] [PubMed] [Google Scholar]
- Dias BC, Souza E, Hara MR, Willemart RH, 2014. Intense leg tapping behavior by the harvestman Mischonyx cuspidatus (Gonyleptidae): an undescribed defensive behavior in Opiliones? J Arachnol 42:123–125. [Google Scholar]
- Edmunds M, 1974. Defence in Animals: A Survey of Anti-Predator Defences. Harlow: Longman. [Google Scholar]
- Eisner T, Rossini C, González A, Eisner M, 2004. Chemical defense of an opilionid Acanthopachylus aculeatus. J Exp Biol 207:1313–1321. [DOI] [PubMed] [Google Scholar]
- Emlen DJ, 2008. The evolution of animal weapons. Annu Rev Ecol Evol S 39:387–413. [Google Scholar]
- Emlen DJ, 2014. Animal Weapons: The Evolution of Battle. New York: Henry Holt and Company. [Google Scholar]
- Gnaspini P, Hara MR, 2007. Defense mechanisms In: Pinto-da-Rocha R, Machado G, Giribet G, editors. Harvestmen: The Biology of Opiliones .Cambridge: Harvard University Press; 374–399. [Google Scholar]
- Gyssels FG, Stoks R, 2005. Threat-sensitive responses to predator attacks in a damselfly. Ethology 111:411–423. [Google Scholar]
- Hara MR, Cavalheiro AJ, Gnaspini P, Santos DY, 2005. A comparative analysis of the chemical nature of defensive secretions of Gonyleptidae (Arachnida: opiliones: laniatores). Biochem Syst Eco 33:1210–1225. [Google Scholar]
- Helfman GS, 1989. Threat-sensitive predator avoidance in damselfish-trumpetfish interactions. Behav Ecol Sociobiol 24:47–58. [Google Scholar]
- Ibáñez A, López P, Martín J, 2014. Inter-individual variation in antipredator hiding behavior of Spanish Terrapins depends on sex, size, and coloration. Ethology 120:742–752. [Google Scholar]
- Irschick DJ, Meyers JJ, Husak JF, Le Galliard JF, 2008. How does selection operate on whole–organism functional performance capacities? A review and synthesis. Evol Eco Res 10:177–196. [Google Scholar]
- Machado G, Carrera PC, Pomini AM, Marsaioli AJ, 2005. Chemical defense in harvestmen (Arachnida, Opiliones): do benzoquinone secretions deter invertebrate and vertebrate predators? J Chem Ecol 31:2519–2539. [DOI] [PubMed] [Google Scholar]
- Machado G, Pomini AM, 2008. Chemical and behavioral defenses of the neotropical harvestman Camarana flavipalpi (Arachnida: opiliones). Biochem Syst Ecol 36:369–376. [Google Scholar]
- Machado G, Requena GS, Buzatto BA, Osses F, Rossetto LM, 2004. Five new cases of paternal care in harvestmen (Arachnida: opiliones): implications for the evolution of male guarding in the Neotropical family Gonyleptidae. Sociobiology 44:577–598. [Google Scholar]
- McCullough EL, Miller CW, Emlen DJ, 2016. Why sexually selected weapons are not ornaments. Trends Ecol Evol 31:742–751. [DOI] [PubMed] [Google Scholar]
- Miller DW, Jones AD, Goldston JS, Rowe MP, Rowe AH, 2016. Sex differences in defensive behavior and venom of the striped bark scorpion Centruroides vittatus (Scorpiones: buthidae). Integr Comp Biol 56:1022–1031. [DOI] [PubMed] [Google Scholar]
- Miyatake T, 1997. Functional morphology of the hind legs as weapons for male contests in Leptoglossus australis (Heteroptera: coreidae). J Insect Behav 10:727–735. [Google Scholar]
- Miyatake T, 2001a. Diurnal periodicity of death-feigning in Cylas formicarius (Coleoptera: brentidae). J Insect Behav 14:421–432. [Google Scholar]
- Miyatake T, 2001b. Effects of starvation on death-feigning in adults of Cylas formicarius (Coleoptera: brentidae). Ann Entomol Soc Am 94:612–616. [Google Scholar]
- Miyatake T, Katayama K, Takeda Y, Nakashima A, Sugita A. et al. 2004. Is death-feigning adaptive? Heristable variation in fitness difference of death-feigning behaviour. Proc R Soc Lond B 271:2293–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake T, Nakayama S, Nishi Y, Nakajima S, 2009. Tonically immobilized selfish prey can survive by sacrificing others. Proc R Soc Lond B 276:2763–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazareth TM, Machado G, 2009. Reproductive behavior of Chavesincola inexpectabilis (Opiliones, Gonyleptidae) with description of a new and independently evolved case of paternal care in harvestmen. J Arachnol 37:127–134. [Google Scholar]
- Nazareth TM, Machado G, 2015. Egg production constrains chemical defenses in a Neotropical arachnid. PLoS ONE 10: e0134908.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira W, Elpino-Campos A, Del-Claro K, Machado G, 2004. Behavioral repertory of the neotropical harvestman Ilhaia cuspidata (Opiliones, Gonyleptidae). J Arachnol 32:22–30. [Google Scholar]
- Pinto-da-Rocha R, Giribet G, 2007. Taxonomy In: Pinto-da-Rocha R, Machado G, Giribet G, editors. Harvestmen: The Biology of Opiliones. Cambridge: Harvard University Press; 88–246. [Google Scholar]
- Pomini AM, Machado G, Pinto-da-Rocha R, Macías-Ordóñez R, Marsaioli AJ, 2010. Lines of defense in the harvestman Hoplobunus mexicanus (Arachnida: opiliones): aposematism, stridulation, thanatosis, and irritant chemicals. Biochem Syst Ecol 38:300–308. [Google Scholar]
- Raspotnig G, Schaider M, Stabentheiner E, Leis HJ, Karaman I, 2014. On the enigmatic scent glands of dyspnoan harvestmen (Arachnida, Opiliones): first evidence for the production of volatile secretions. Chemoecology 24:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitze M, Nentwig W, 1991. Comparative investigations into the feeding ecology of six Mantodea species. Oecologia 86:568–574. [DOI] [PubMed] [Google Scholar]
- Santos F, 2007. Ecophysiology In: Pinto-da-Rocha, Machado RG, Giribet G, editors. Harvestmen: The Biology of Opiliones. Cambridge: Harvard University Press; 473–488. [Google Scholar]
- Segalerba A, Toscano-Gadea CA, 2016. Description of the defensive behaviour of four Neotropical harvestmen (Laniatores: gonyleptidae) against a synchronic and sympatric wolf spider (Araneae: lycosidae). Arachnology 17:52–58. [Google Scholar]
- Segovia JMG, Del-Claro K, Willemart RH, 2015a. Defences of a Neotropical harvestman against different levels of threat by the recluse spider. Behaviour 152:757–773. [Google Scholar]
- Segovia JMG, De-Claro K, Willemart RH, 2015b. Delicate fangs, smart killing: the predation strategy of the recluse spider. Anim Behav 101:169–177. [Google Scholar]
- Segovia JMG, Hara MR, Pagoti GF, Sannomiya M, Santos DY. et al. 2015c. The scent glands of the Neotropical harvestman Discocyrtus pectnifemur: morphology, Behavior and Chemistry. J Chem Ecol 41:716–723. [DOI] [PubMed] [Google Scholar]
- Shettleworth SJ, 2010. Cognition, Evolution, and Behavior. 2nd edn. New York: Oxford University Press. [Google Scholar]
- Souza E, Willemart RH, 2011. Harvest-ironman: heavy armature, and not its defensive secretions, protects a harvestman against a spider. Anim Behav 81:127–133. [Google Scholar]
- Willemart RH, Osses F, Chelini MC, Macias-Ordonez R, Machado G, 2009. Sexually dimorphic legs in a neotropical harvestman (Arachnida, Opiliones): ornament or weapon? Behav Process 80:51–59. [DOI] [PubMed] [Google Scholar]
- Worthington AM, Swallow JG, 2010. Gender differences in survival and antipredatory behavior in stalk-eyed flies. Behav Ecol 21:759–766. [Google Scholar]
- Zatz C, Werneck RM, Macías-Ordóñez R, Machado G, 2011. Alternative mating tactics in dimorphic males of the harvestman Longiperna concolor (Arachnida: opiliones). Behav Ecol Sociobiol 65:995–1005. [Google Scholar]