Key Points
Disease-free survival is higher with myeloablative regimens for patients in their third to fifth decade.
Beyond the fifth decade, low-dose total body irradiation regimens offset mortality associated with transplant procedure.
Abstract
In the absence of prospective studies that examine the effect of conditioning regimen intensity after T-cell–replete haploidentical transplant for acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and myelodysplastic syndrome (MDS), a retrospective cohort analysis was performed. Of the 1325 eligible patients (AML, n = 818; ALL, n = 286; and MDS, n = 221), 526 patients received a myeloablative regimen and 799 received a reduced-intensity regimen. Graft-versus-host disease prophylaxis was uniform with posttransplant cyclophosphamide, a calcineurin inhibitor, and mycophenolate mofetil. The primary end point was disease-free survival. Cox regression models were built to study the effect of conditioning regimen intensity on transplant outcomes. For patients aged 18 to 54 years, disease-free survival was lower (hazard ratio [HR], 1.34; 42% vs 51%; P = .007) and relapse was higher (HR, 1.51; 44% vs 33%; P = .001) with a reduced-intensity regimen compared with a myeloablative regimen. Nonrelapse mortality did not differ according to regimen intensity. For patients aged 55 to 70 years, disease-free survival (HR, 0.97; 37% vs 43%; P = .83) and relapse (HR, 1.32; 42% vs 31%; P = .11) did not differ according to regimen intensity. Nonrelapse mortality was lower with reduced-intensity regimens (HR, 0.64; 20% vs 31%; P = .02). Myeloablative regimens are preferred for AML, ALL, and MDS; reduced-intensity regimens should be reserved for those unable to tolerate myeloablation.
Visual Abstract
Introduction
In recent years, data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) and the European Society for Blood and Marrow Transplantation (EBMT) confirm increasing numbers of haploidentical transplants.1,2 In the United States, T-cell–replete haploidentical donor transplant utilizing posttransplant cyclophosphamide with a calcineurin inhibitor and mycophenolate mofetil for graft-versus-host disease (GVHD) prophylaxis has emerged as a safe and effective alternative for those without an HLA-matched sibling.3-6 The original experience of the Hopkins group for haploidentical transplant with posttransplant cyclophosphamide for GVHD prophylaxis used a conditioning regimen that included low-dose total body irradiation (TBI; 200 cGy) with cyclophosphamide and fludarabine.3,4 The Hopkins approach resulted in low GVHD and nonrelapse mortality, but relapse was higher, which negated a survival advantage compared with other alternative donor types.6,7 In recent years, others have tested myeloablative conditioning regimens for T-cell–replete haploidentical transplant for adults with hematologic malignancy and were able to document feasibility and effectiveness.8-11
There have been several retrospective studies on HLA-matched sibling and unrelated donor transplants that have compared transplant outcomes after myeloablative and reduced-intensity conditioning regimens for acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and myelodysplastic syndrome (MDS).12-16 All except one report failed to show differences in disease-free or overall survival.16 That report compared specific individual myeloablative or reduced-intensity conditioning regimens and concluded the modest reduction in nonrelapse mortality that was associated with reduced-intensity busulfan and fludarabine did not negate the high relapse associated with this regimen. Furthermore, the results of 3 recent randomized clinical trials17-19 that compared myeloablative vs reduced-intensity regimens for AML and MDS also yielded mixed results, with only one trial18 reporting higher relapse-free survival with myeloablative conditioning regimens; the difference in overall survival between regimens did not reach statistical significance, however.
In the absence of a randomized trial or a large retrospective report on the association between conditioning regimen intensity for T-cell–replete haploidentical transplant, the goal of the current analyses was to study the broad question of “myeloablative vs reduced-intensity” conditioning regimens for adults with AML, ALL, and MDS undergoing T-cell–replete haploidentical transplant in the United States.
Patients and methods
Patients
The CIBMTR is a group of >400 transplant centers worldwide that contribute data prospectively on consecutive transplants performed at each individual center, and patients are followed up until death or lost to follow-up. Seventy-eight of 195 transplant centers in the United States contributed data for the current analyses. Transplants occurred between 2008 and 2016. Eligible patients were aged 18 to 70 years with AML, ALL, or MDS and received T-cell–replete bone marrow or peripheral blood from a haploidentical relative (mismatched at ≥2 HLA loci). All patients received uniform GVHD prophylaxis: posttransplant cyclophosphamide with a calcineurin inhibitor and mycophenolate mofetil. Transplant conditioning regimens were grouped as myeloablative or reduced intensity based on published criteria.20 Regimens were considered myeloablative when the TBI dose was >800 cGy, busulfan >8 mg/kg (oral) or >6 mg/kg (IV), melphalan >150 mg/m2, or melphalan 140 mg/m2 with thiotepa. Lower dose TBI dose (200 or 300 cGy), melphalan ≤140 mg/m2 (administered without another alkylating agent), and busulfan ≤8 mg/kg (oral) or ≤6 mg/kg (IV) were considered reduced intensity. Excluded were T-cell–depleted transplants (CD34-selected peripheral blood [n = 325] or antithymocyte globulin or alemtuzumab [n = 52]) and patients aged >70 years (n = 89). All patients provided written informed consent for research. The institutional review board of the National Marrow Donor Program approved the study.
End points
The primary end point was disease-free survival. Relapse or death were considered as events. Relapse was defined as morphologic, cytogenetic, or molecular disease recurrence, and nonrelapse mortality was defined as death in remission. Neutrophil recovery was defined as achieving an absolute neutrophil count (ANC) ≥0.5 × 109/L for 3 consecutive days and platelets ≥20 × 109/L unsupported by transfusion for 7 days. Primary and secondary graft failures were considered as a single outcome. Primary graft failure was defined as failure to achieve an ANC ≥0.5 × 109/L for 3 consecutive days or donor chimerism <5%. Secondary graft failure was defined as initial donor engraftment followed by graft loss, evidenced by a persistent decline in the ANC (<0.5 × 109/L) or loss of donor chimerism to <5% or a second transplant in patients with documented clinical remission.21 Acute GVHD grade II to IV and chronic GVHD were based on reports from each transplant center using standard criteria.22,23 Death from any cause was considered an event for overall survival. Surviving patients were censored at last follow-up.
Statistical methods
Differences in patient, disease, and transplant characteristics between the treatment groups (myeloablative or reduced intensity) were compared by using the χ2 statistic for categorical variables. The probabilities of disease-free and overall survival were calculated by using the Kaplan-Meier estimator.24 The probabilities of neutrophil and platelet recovery, acute and chronic GVHD, nonrelapse mortality, and relapse were calculated by using the cumulative incidence estimator to accommodate competing risks.25 Cox regression models were built to study the effect of conditioning regimen intensity (myeloablative vs reduced intensity) on disease-free survival, overall survival, grade II to IV acute GVHD, chronic GVHD, relapse, and nonrelapse mortality.26 Age at transplantation was a significant predictor for disease-free survival, with differences seen between patients aged 18 to 54 years and 55 to 70 years. Analyses of transplant outcomes were therefore studied separately for the 2 age groups. Other factors tested for their effect on transplant outcomes included sex, performance score, comorbidity score, cytomegalovirus (CMV) serostatus, disease, disease risk index, graft type, and transplant period. All variables that attained P < .05 were held in the final multivariate model with the exception of the variable for regimen intensity, which was held in all steps of model building and the final model regardless of level of significance. The effect of transplant center on survival was tested by using the frailty model.27 All P values are 2-sided, and analyses were performed by using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Patient, disease, and transplant characteristics
The characteristics of patients, their disease, and transplant according to conditioning regimen intensity (myeloablative, n = 526; reduced intensity, n = 799) are shown in Table 1. The predominant myeloablative regimens were non-TBI containing (58%), and most of these regimens included busulfan and cyclophosphamide (14%), busulfan and fludarabine (13%), or busulfan, cyclophosphamide, and fludarabine (22%). Melphalan and fludarabine with or without thiotepa accounted for 9% of myeloablative regimens. The predominant reduced-intensity regimen was low-dose TBI (200 cGy), cyclophosphamide, and fludarabine (84%). Patients aged 18 to 54 years were more likely to receive myeloablative regimens (381 of 689 [55%]) compared with reduced-intensity regimens (308 of 689 [45%]). Use of myeloablative regimens decreased with increasing age. Among patients aged 55 to 70 years, 23% (145 of 636) received myeloablative regimens compared with 77% (491 of 636) who received reduced-intensity regimens. Of the 145 patients aged 55 to 70 years who received myeloablative regimens, 65 (45%) were aged 55 to 59 years, 63 (43%) were aged 60 to 65 years, and 17 (12%) were aged 66 to 70 years. The corresponding distribution for 491 patients who received reduced-intensity regimens were 120 (24%), 210 (43%), and 161 (33%), respectively.
Table 1.
Patient, disease, and transplant characteristics
| Variable | Myeloablative | Reduced intensity | P |
|---|---|---|---|
| No. of patients | 526 | 799 | |
| Age, n (%), y | <.001 | ||
| 18-54 | 381 (72) | 308 (39) | |
| 55-70 | 145 (28) | 491 (61) | |
| Sex, n (%) | .03 | ||
| Male | 279 (53) | 472 (59) | |
| Female | 247 (47) | 327 (41) | |
| Performance score, n (%) | .02 | ||
| 90-100 | 267 (51) | 460 (58) | |
| ≤80 | 243 (46) | 307 (38) | |
| Not reported | 16 (3) | 32 (4) | |
| Comorbidity score, n (%) | .15 | ||
| 0-2 | 288 (55) | 392 (49) | |
| ≥3 | 238 (45) | 407 (51) | |
| CMV serostatus, n (%) | .70 | ||
| Negative | 157 (30) | 250 (31) | |
| Positive | 364 (69) | 544 (68) | |
| Not reported | 5 (<1) | 5 (<1) | |
| Disease, n (%) | <.001 | ||
| AML | 329 (63) | 489 (61) | |
| ALL | 136 (26) | 150 (19) | |
| MDS | 61 (12) | 160 (20) | |
| Disease status: AML/ALL, n (%) | <.001 | ||
| First complete remission | 235 (45) | 387 (48) | |
| Second complete remission | 114 (22) | 146 (18) | |
| Relapse | 116 (22) | 106 (13) | |
| Disease status: MDS, n (%) | <.001 | ||
| RA/RARS/RCMD | 9 (2) | 21 (3) | |
| RAEB-1/RAEB-2 | 50 (10) | 135 (17) | |
| Not reported | 2 (<1) | 4 (<1) | |
| Cytogenetic risk: AML, n (%) | .80 | ||
| Favorable | 28 (9) | 33 (7) | |
| Intermediate | 261 (79) | 398 (81) | |
| Poor | 28 (9) | 39 (8) | |
| Not reported | 12 (3) | 19 (4) | |
| Cytogenetic risk: ALL, n (%) | .98 | ||
| Normal | 25 (18) | 28 (19) | |
| Poor | 65 (48) | 73 (49) | |
| Not reported | 46 (34) | 49 (33) | |
| Cytogenetic risk: MDS, n (%) | .95 | ||
| Intermediate | 36 (59) | 91 (57) | |
| Poor | 16 (26) | 45 (28) | |
| Not reported | 9 (15) | 24 (15) | |
| Disease risk index, n (%) | .01 | ||
| Low | 25 (5) | 26 (3) | |
| Intermediate | 261 (50) | 463 (58) | |
| High/very high | 217 (41) | 268 (34) | |
| Not reported | 23 (4) | 42 (5) | |
| Myeloablative regimens, n (%) | NA | ||
| TBI/fludarabine | 171 (33) | … | |
| TBI/other agents | 51 (10) | … | |
| Busulfan/cyclophosphamide | 76 (14) | … | |
| Busulfan/fludarabine | 68 (13) | … | |
| Busulfan/cyclophosphamide/fludarabine | 114 (22) | … | |
| Melphalan/fludarabine ± thiotepa | 46 (9) | … | |
| Reduced-intensity regimens, n (%) | |||
| TBI/busulfan/fludarabine | … | 17 (2) | |
| TBI/cyclophosphamide/fludarabine | … | 668 (84) | |
| TBI/melphalan/fludarabine | … | 42 (5) | |
| Busulfan/fludarabine | … | 9 (1) | |
| Melphalan/fludarabine | … | 63 (17) | |
| Graft type, n (%) | <.001 | ||
| Bone marrow | 181 (34) | 464 (58) | |
| Peripheral blood | 345 (66) | 335 (42) | |
| GVHD prophylaxis | |||
| Posttransplant cyclophosphamide/calcineurin inhibitor/mycophenolate | 526 (100) | 799 (100) | NA |
| Transplant period, n (%) | .28 | ||
| 2008-2012 | 94 (18) | 162 (20) | |
| 2013-2016 | 432 (82) | 637 (80) |
NA, not applicable; RA, refractory anemia; RAEB, refractory anemia with excess blasts; RARS, refractory anemia with ringed sideroblast; RCMD, refractory anemia with multilineage dysplasia.
Both treatment groups received uniform GVHD prophylaxis with posttransplant cyclophosphamide, a calcineurin inhibitor, and mycophenolate mofetil. Compared with recipients of reduced-intensity regimens, recipients of myeloablative regimens were younger (median age, 45 vs 59 years; P < .001) and more likely to be female, have a performance score ≤80, have ALL, be in relapse at transplantation, have a high or very high disease risk index, and receive peripheral blood. There were no differences between treatment groups regarding comorbidity score or CMV serostatus. The median follow-up of recipients of myeloablative and reduced-intensity regimens were 24 months (6-62) and 24 months (3-97), respectively.
Disease-free survival
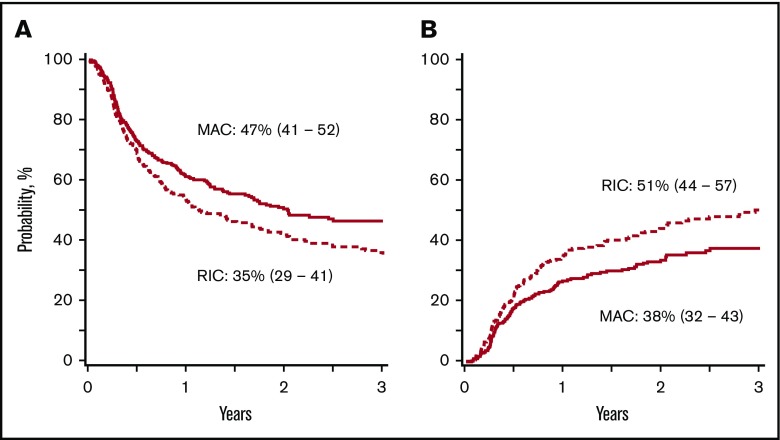
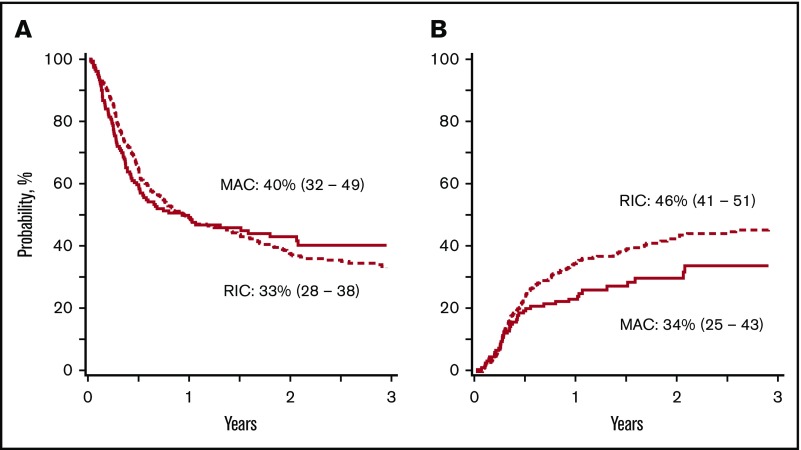
In patients aged 18 to 54 years, disease-free survival was lower with reduced-intensity regimens, after adjusting for comorbidity score, graft type, and disease risk index (composite of cytogenetic risk and disease status at transplantation)28 (Tables 2 and 3; Figure 1A). Disease-free survival did not differ according to conditioning regimen intensity in patients aged 55 to 70 years (Figure 2A). Regardless of patient age, disease-free survival was not different between non-TBI–containing and TBI-containing myeloablative regimens (hazard ratio [HR], 1.13; 95% confidence interval [CI], 0.88-1.45; P = .35) and reduced-intensity regimens (HR, 1.23; 95% CI, 0.91-1.65; P = .17).
Table 2.
Effect of conditioning regimen intensity on disease-free survival, relapse, nonrelapse mortality, and survival
| Outcome | No. of events/evaluable | HR (95% CI) | P |
|---|---|---|---|
| Disease-free survival* | |||
| Age, 18-54 y | |||
| Myeloablative | 183/379 | 1.00 | |
| Reduced intensity | 184/306 | 1.34 (1.08-1.65) | .007 |
| Age, 55-70 y | |||
| Myeloablative | 87/144 | 1.00 | |
| Reduced intensity | 295/489 | 0.97 (0.76-1.24) | .83 |
| Relapse† | |||
| Age, 18-54 y | |||
| Myeloablative | 122/379 | 1.00 | |
| Reduced intensity | 140/306 | 1.51 (1.17-1.94) | .001 |
| Age, 55-70 y | |||
| Myeloablative | 43/144 | 1.00 | |
| Reduced intensity | 201/489 | 1.32 (0.94-1.84) | .11 |
| Nonrelapse mortality‡ | |||
| Age, 18-54 y | |||
| Myeloablative | 61/379 | 1.00 | |
| Reduced intensity | 44/306 | 0.98 (0.66-1.45) | .92 |
| Age, 55-70 y | |||
| Myeloablative | 44/144 | 1.00 | |
| Reduced intensity | 94/489 | 0.64 (0.44-0.92) | .02 |
| Overall survival§ | |||
| Age, 18-54 y | |||
| Myeloablative | 154/381 | 1.00 | |
| Reduced intensity | 138/308 | 1.13 (0.90-1.43) | .30 |
| Age, 55-70 y | |||
| Myeloablative | 82/145 | 1.00 | |
| Reduced intensity | 267/491 | 0.86 (0.67-1.32) | .25 |
Lower disease-free survival for patients with comorbidity score ≥3 compared with ≤2 (HR, 1.21; 95% CI, 1.05-1.40; P = .001) and high disease risk index compared with low disease risk index (HR, 2.04; 95% CI, 1.33-3.14; P = .001) and intermediate disease risk index (HR, 2.03; 95% CI, 1.74-2.37; P < .001). Graft type was not associated with an increased risk of disease-free survival (HR, 0.93 95% CI, 0.80-1.08; P = .34).
Higher relapse for patients with high disease risk index (HR, 2.45; 95% CI, 1.42-4.24; P = .001). Relapse was lower for patients with MDS compared with AML (HR, 0.68; 95% CI, 0.52-0.89; P = .004) and transplant of peripheral blood compared with bone marrow (HR, 0.82; 95% CI, 0.68-0.98; P = .03). Comorbidity score ≥3 was not associated with an increased risk of relapse (HR, 1.06; 95% CI, 0.89-1.27; P = .51).
Higher nonrelapse mortality for patients with comorbidity score ≥3 compared with ≤2 (HR, 1.59; 95% CI, 1.23-2.07; P = .0005) and high disease risk index compared with intermediate disease risk index (HR, 1.80; 95% CI, 1.37-2.37; P < .0001).
Lower overall survival for patients with comorbidity score ≥3 compared with ≤2 (HR, 1.27; 95% CI, 1.08-1.49; P = .003), CMV seropositivity (HR, 1.22; 95% CI, 1.02-1.45; P = .03), AML compared with ALL (HR, 1.40; 95% CI, 1.13-1.85; P = .002), and high disease risk index compared with low disease risk index (HR, 2.37; 95% CI, 1.42-3.94; P = .001) and intermediate disease risk index (HR, 2.00; 95% CI, 1.70-2.36; P < .001).
Table 3.
Three-year adjusted probabilities of disease-free survival, relapse, nonrelapse mortality, overall survival, chronic GVHD, and day 100 acute grade II to IV acute GVHD
| Outcome | Myeloablative regimen (95% CI), % | Reduced-intensity regimen (95% CI), % | P |
|---|---|---|---|
| Disease-free survival | |||
| Age, 18-54 y | 47 (41-52) | 35 (29-41) | .009 |
| Age, 55-70 y | 40 (32-49) | 33 (28-38) | .15 |
| Relapse | |||
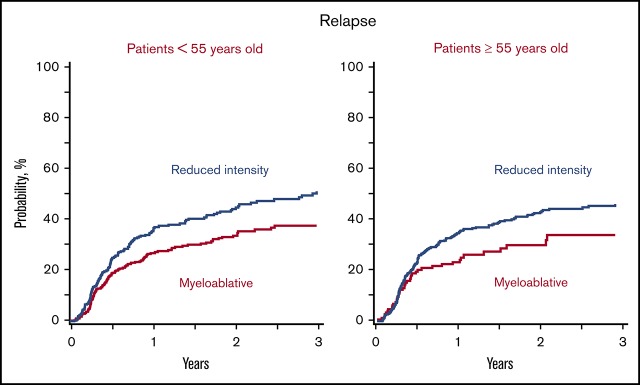
| Age, 18-54 y | 38 (32-43) | 51 (44-57) | .003 |
| Age, 55-70 y | 34 (25-43) | 46 (41-51) | .03 |
| Nonrelapse mortality | |||
| Age, 18-54 y | 18 (14-22) | 17 (12-22) | .81 |
| Age, 55-70 y | 30 (22-38) | 24 (19-30) | .26 |
| Overall survival | |||
| Age, 18-54 y | 54 (49-60) | 49 (42-55) | .19 |
| Age, 55-70 y | 42 (33-50) | 38 (33-43) | .48 |
| Acute grade II-IV GVHD | |||
| Age, 18-54 y | 24 (20-28) | 20 (15-25) | .23 |
| Age, 55-70 y | 23 (16-30) | 20 (17-24) | .52 |
| Chronic GVHD | |||
| Age, 18-54 y | 38 (29-47) | 38 (33-43) | .97 |
| Age, 55-70 y | 26 (19-34) | 28 (23-32) | .75 |
Figure 1.
Disease-free survival and relapse in patients aged 18 to 54 years. (A) The 3-year adjusted probability of disease-free survival after a myeloablative (MAC) regimen (47%; 95% CI, 41-52) and after a reduced-intensity (RIC) regimen (35%; 95% CI, 29-41) (P = .009). (B) The 3-year adjusted probability of relapse after a MAC regimen (38%; 95% CI, 32-43) and after an RIC regimen (51%; 95% CI, 44-57) (P = .003).
Figure 2.
Disease-free survival and relapse in patients aged 55 to 70 years. (A) The 3-year adjusted probability of disease-free survival after a myeloablative (MAC) regimen (40%; 95% CI, 32-49) and after a RIC regimen (33%; 95% CI, 28-38) (P = .15). (B) The 3-year adjusted probability of relapse after a MAC regimen (34%; 95% CI, 25-43) and after an RIC regimen (46%; 95% CI, 41-51) (P = .03).
Relapse
Relapse was higher with reduced-intensity regimens in patients aged 18 to 54 years after adjusting for comorbidity index, disease, disease risk index, and graft type (Tables 2 and 3; Figure 1B). In patients aged 55 to 70 years, relapse risks did not differ according to conditioning regimen until several years after transplantation (Figure 2B). The 3-year incidence of relapse was higher after reduced-intensity regimens adjusted for comorbidity index, disease, disease risk index, and graft type. Regardless of age, relapse did not differ between non-TBI–containing and TBI-containing myeloablative regimens (HR, 0.98; 95% CI, 0.72-1.35; P = .92) or reduced-intensity regimens (HR, 0.90; 95% CI, 0.60-1.33; P = .59).
Nonrelapse mortality
Nonrelapse mortality did not differ according to conditioning regimen intensity in patients aged 18 to 54 years but was lower with reduced-intensity regimens in older patients (Table 2). The 3-year cumulative incidence data for nonrelapse mortality adjusted for comorbidity score and disease risk index are presented in Table 3. Nonrelapse mortality did not differ between TBI-containing and non-TBI–containing myeloablative regimens (HR, 1.39; 95% CI, 0.91-2.11; P = .13). Nonrelapse mortality was higher with reduced-intensity non-TBI–containing regimens compared with TBI-containing regimens (HR, 2.14; 95% CI, 1.36-3.37; P = .03).
Overall survival
Overall survival did not differ according to conditioning regimen intensity after adjusting for disease, comorbidity score, CMV seropositivity, and disease risk index (Tables 2 and 3). A total of 236 (45%) of 526 and 405 (51%) of 799 recipients of myeloablative and reduced-intensity transplants, respectively, died. The causes of death did not differ according to conditioning regimen intensity (P = .35). Recurrent disease was the predominant cause of death, accounting for 54% and 61% after myeloablative and reduced-intensity transplants. Other frequent causes of death included infection (13% vs 14%), GVHD (7% vs 5%), and organ failure (8% vs 8%) after myeloablative and reduced-intensity transplants.
Hematopoietic recovery
The median times to neutrophil and platelet recovery with myeloablative and reduced-intensity conditioning regimens did not differ: 17 vs 17 days for neutrophils and 27 vs 27 days for platelets. The day 28 incidence of neutrophil recovery was 89% (95% CI, 86-92) and 90% (95% CI, 88-92) after myeloablative and reduced-intensity conditioning regimens, respectively (P = .54). The corresponding day 100 incidence of platelet recovery was 87% (95% CI, 84-90) and 88% (95% CI, 86-90) (P = .73). The 1-year incidence of graft failure with myeloablative and reduced-intensity conditioning regimens did not differ: 9% (95% CI, 7-12) and 12% (95% CI, 9-14) (P = .13). There was no interaction between conditioning intensity and graft type on graft failure (P = .25). Only 27 (18%) of 147 patients with graft failure are alive. Most graft failures occurred early; 52 patients received a second infusion (44 transplants and 8 donor leukocyte infusion). Median time to second infusion was 3 months after the first transplant. Only 23 (44%) of 52 patients are alive. Most patients (n = 39) received the second infusion from the same donor as for their first transplant. Fourteen patients who received a myeloablative regimen for their first transplant received a reduced-intensity regimen for their second transplant. Seven who received a reduced-intensity regimen for their first transplant received a myeloablative regimen for their second transplant.
Graft-versus-host disease
There were no differences in grade II to IV acute GVHD (HR, 1.01; 95% CI, 0.79-1.29; P = .94) and chronic GVHD (HR, 0.82; 95% CI, 0.62-1.07; P = .14) according to regimen intensity for patients aged 18 to 54 years. Similarly, grade II to IV acute GVHD (HR, 0.88; 95% CI, 0.64-1.07; P = .14) and chronic GVHD (HR, 0.86; 95% CI, 0.59-1.26; P = .43) did not differ according to regimen intensity for older patients. Independent of age and regimen intensity, compared with transplantation of bone marrow, transplantation of peripheral blood was associated with higher grade II to IV acute GVHD (HR, 1.49; 95% CI, 1.23-1.79; P < .0001) and chronic GVHD (HR, 2.19; 95% CI, 1.76-2.73; P < .0001). The adjusted day 100 incidence of grade II to IV acute GVHD and 3-year incidence of chronic GVHD are presented in Table 3.
Subset analysis
A subset analysis limited to patients with myeloid malignancies was conducted (Table 4). Consistent with the main analysis, in patients aged 18 to 54 years, disease-free survival was higher and relapse was lower after myeloablative regimens. However, there was a survival advantage with myeloablative regimens that was not recorded for the whole cohort (AML, ALL, and MDS). In patients aged 55 to 70 years, consistent with the main analysis, there were no differences recorded between myeloablative and reduced-intensity regimens.
Table 4.
Effect of conditioning regimen intensity on disease-free survival, relapse, nonrelapse mortality, and survival: AML and MDS only
| Outcome | No. of events/evaluable | HR (95% CI) | P |
|---|---|---|---|
| Disease-free survival* | |||
| Age, 18-54 y | |||
| Myeloablative | 121/258 | 1.00 | |
| Reduced intensity | 130/212 | 1.44 (1.12-1.86) | .004 |
| Age, 55-70 y | |||
| Myeloablative | 81/130 | 1.00 | |
| Reduced intensity | 265/433 | 0.94 (0.73-1.22) | .66 |
| Relapse† | |||
| Age, 18-54 y | |||
| Myeloablative | 81/258 | 1.00 | |
| Reduced intensity | 93/212 | 1.54 (1.14-2.10) | .005 |
| Age, 55-70 y | |||
| Myeloablative | 41/130 | 1.00 | |
| Reduced intensity | 181/433 | 1.25 (0.89-1.76) | .21 |
| Nonrelapse mortality‡ | |||
| Age, 18-54 y | |||
| Myeloablative | 40/258 | 1.00 | |
| Reduced intensity | 37/212 | 1.24 (0.79-2.00) | .36 |
| Age, 55-70 y | |||
| Myeloablative | 40/130 | 1.00 | |
| Reduced intensity | 84/433 | 0.63 (0.43-0.93) | .02 |
| Overall survival§ | |||
| Age, 18-54 y | |||
| Myeloablative | 105/259 | 1.00 | |
| Reduced intensity | 110/214 | 1.35 (1.04-1.77) | .027 |
| Age, 55-70 y | |||
| Myeloablative | 76/131 | 1.00 | |
| Reduced intensity | 241/435 | 0.84 (0.65-1.09) | .18 |
Lower disease-free survival for patients with comorbidity score ≥3 compared with ≤2 (HR, 1.26; 95% CI, 1.07-1.48; P = .0049) and high disease risk index compared with low disease risk index (HR, 1.97; 95% CI, 1.27-3.03; P = .002) and intermediate disease risk index (HR, 1.94; 95% CI, 1.64-2.31; P < .001). Graft type was not associated with an increased risk of disease-free survival (HR, 1.09; 95% CI, 0.93-1.30; P = .27).
Higher relapse for patients with high disease risk index (HR, 2.13; 95% CI, 1.72-2.64; P < .001). Relapse was lower for patients with MDS compared with AML (HR, 0.67; 95% CI, 0.52-0.88; P = .004) and transplant of peripheral blood compared with bone marrow (HR, 0.80; 95% CI, 0.65-0.98; P = .03). Comorbidity score ≥3 was not associated with an increased risk of relapse (HR, 1.08; 95% CI, 0.89-1.32; P = .43).
Higher nonrelapse mortality for patients with comorbidity score ≥3 compared with ≤2 (HR, 1.71; 95% CI, 1.28-2.82; P = .0003) and high disease risk index compared with intermediate disease risk index (HR, 1.63; 95% CI, 1.19-2.22; P = .0019).
Lower overall survival for patients with comorbidity score ≥3compared with ≤2 (HR, 1.31; 95% CI, 1.10-1.56; P = .002), CMV seropositivity (HR, 1.34; 95% CI, 1.11-1.62; P = .03), and high disease risk index compared with low disease risk index (HR, 2.29; 95% CI, 1.37-3.82; P = .001) and intermediate disease risk index (HR, 1.87; 95% CI, 1.56-2.24; P < .001).
Discussion
This retrospective analysis recorded lower relapse and higher disease-free survival with myeloablative regimens in patients aged 18 to 54 years when classifying regimen intensity broadly as myeloablative or reduced intensity. In the subset of patients with myeloid malignancies, higher overall survival was recorded with myeloablative regimens in patients aged 18 to 54 years. In older patients (age 55-70 years), differences in relapse incidence were recorded beyond 2 years after transplant but with no differences in disease-free survival. We hypothesize that higher nonrelapse mortality (although not statistically significant) associated with myeloablative regimens occurred primarily early posttransplant and negated any potential advantage for disease-free survival in older patients. Our findings are in keeping with those reported from a recent North American trial that randomized patients with myeloid malignancies undergoing HLA-matched sibling or unrelated donor transplant to receive myeloablative or reduced-intensity conditioning regimens.18 Consistent with the findings of randomized trials on regimen intensity for HLA-matched sibling or unrelated donor transplants, the current analysis also did not record significant differences in survival except in the subset of patients with myeloid malignancies.17-19 The modest follow-up of patients in our study is perhaps insufficient for exploring the effects of relapse on survival when multiple salvage treatment modalities are available.
Our findings differ from a retrospective report from the EBMT.29 That report did not show differences in haploidentical transplant outcomes according to conditioning regimen intensity. However, there are several differences between the current analyses and the EBMT report that explain the differences between the 2 studies. Most notably, <30% of transplants in the EBMT analysis were performed by using posttransplant cyclophosphamide for GVHD prophylaxis, approximately one-half of transplants used in vivo T-cell depletion, and the study population was about one-half that of the current study, which precluded an analysis according to patient age groups (ie, ability to examine for differences within relatively younger and older populations). Consistent with published reports, higher comorbidity index and poor risk disease characteristics were associated with worse outcomes in the current analysis, underscoring the importance of patient selection for transplantation. We failed to record significant differences in hematopoietic recovery or graft failure according to conditioning regimen intensity. Similarly, acute and chronic GVHD also did not differ according to conditioning regimen intensity. In fact, acute and chronic GVHD risks were mitigated by graft type. Consistent with that reported by others, the recorded higher GVHD risks with peripheral blood did not result in higher nonrelapse mortality or lower survival.30
A significant weakness of our study is the fact that we performed a retrospective comparison of conditioning regimen intensity on transplant outcomes. We have assumed that conditioning regimens were chosen based on a number of factors, including tolerability of the regimen, transplant center preference, and/or patient and physician preference. As such, even though we performed carefully controlled analyses adjusting for known risk factors, there may be unknown or unmeasured risk factors that may have influenced outcomes. We used the broad category of myeloablation and reduced intensity rather than individual regimens, which prevented us from examining for differences between regimens. Lower nonrelapse mortality with low-dose TBI-containing regimens merits further study: transplants as a potentially curative option are increasingly offered to older and less fit patients who are unlikely candidates for myeloablation. Strengths of our study include the large numbers of patients and our ability to study conditioning regimen intensities in younger and older patients as well as examine for differences between TBI-containing and non-TBI–containing regimens among the broad categories of myeloablative and reduced-intensity regimens. Our findings are in keeping with those reported from randomized trials that addressed conditioning regimen intensity after HLA-matched related and unrelated donor transplant.17-19 Our findings support the use of myeloablative conditioning for adults with acceptable comorbidity scores, regardless of their age, to lower relapse risks and improve disease-free survival for haploidentical transplant with posttransplant cyclophosphamide.
Acknowledgments
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases, National Institutes of Health; 5U10HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute, National Institutes of Health; a contract (HHSH250201200016C) with the Health Resources and Services Administration (US Department of Health and Human Services); and grants N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, the Health Resources and Services Administration, or any other agency of the US Government.
Authorship
Contribution: S.R.S., A.S.M., N.N.S., G.F., M.-J.Z., and M.E. designed the study; A.S.M. prepared the study file for analyses and analyzed the data; S.R.S., A.S.M., N.N.S., G.F., M.-J.Z., and M.E. summarized and interpreted the findings; S.R.S. drafted the manuscript; A.S.M., N.N.S., G.F., M.M.A.M., K.K.B., A.B., N.B., J.B.M., C.G.B., Z.D., R.E.C., E.J.F., M.H., P.H., C.G.K., J.P.M., I.K.M., S.O.C., M.C.P., V.R., R.R., S.S.P., S.V., E.K.W., J.R.W., M.-J.Z., and M.E critically reviewed and edited the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Scott Solomon, The Blood and Marrow Transplant Program, Northside Hospital, 5670 Peachtree Dunwoody Rd NE, Suite 1000, Atlanta, GA 30342; e-mail: ssolomon@bmtga.com.
References
- 1.D’Souza A, Lee S, Zhu X, Pasquini M. Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2017;23(9):1417-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Passweg JR, Baldomero H, Bader P, et al. . Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017;52(6):811-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Donnell PV, Luznik L, Jones RJ, et al. . Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8(7):377-386. [DOI] [PubMed] [Google Scholar]
- 4.Luznik L, O’Donnell PV, Symons HJ, et al. . HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bashey A, Zhang X, Sizemore CA, et al. . T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31(10):1310-1316. [DOI] [PubMed] [Google Scholar]
- 6.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. . Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunstein CG, Fuchs EJ, Carter SL, et al. ; Blood and Marrow Transplant Clinical Trials Network . Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raiola AM, Dominietto A, Ghiso A, et al. . Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19(1):117-122. [DOI] [PubMed] [Google Scholar]
- 9.Grosso D, Gaballa S, Alpdogan O, et al. . A two-step approach to myeloablative haploidentical transplantation: low nonrelapse mortality and high survival confirmed in patients with earlier stage disease. Biol Blood Marrow Transplant. 2015;21(4):646-652. [DOI] [PubMed] [Google Scholar]
- 10.Solomon SR, Sizemore CA, Sanacore M, et al. . Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant. 2012;18(12):1859-1866. [DOI] [PubMed] [Google Scholar]
- 11.Solomon SR, Sizemore CA, Sanacore M, et al. . Total body irradiation-based myeloablative haploidentical stem cell transplantation is a safe and effective alternative to unrelated donor transplantation in patients without matched sibling donors. Biol Blood Marrow Transplant. 2015;21(7):1299-1307. [DOI] [PubMed] [Google Scholar]
- 12.Marks DI, Wang T, Pérez WS, et al. . The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission. Blood. 2010;116(3):366-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohty M, Labopin M, Volin L, et al. ; Acute Leukemia Working Party of EBMT . Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2010;116(22):4439-4443. [DOI] [PubMed] [Google Scholar]
- 14.Ringdén O, Labopin M, Ehninger G, et al. . Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27(27):4570-4577. [DOI] [PubMed] [Google Scholar]
- 15.Luger SM, Ringdén O, Zhang MJ, et al. . Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47(2):203-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eapen M, Brazauskas R, Hemmer M, et al. . Hematopoietic cell transplant for acute myeloid leukemia and myelodysplastic syndrome: conditioning regimen intensity. Blood Adv. 2018;2(16):2095-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bornhäuser M, Kienast J, Trenschel R, et al. . Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13(10):1035-1044. [DOI] [PubMed] [Google Scholar]
- 18.Scott BL, Pasquini MC, Logan BR, et al. . Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35(11):1154-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kröger N, Iacobelli S, Franke GN, et al. . Dose-reduced versus standard conditioning followed by allogeneic stem-cell transplantation for patients with myelodysplastic syndrome: a prospective randomized phase III study of the EBMT (RICMAC) trial. J Clin Oncol. 2017;35(19):2157-2164. [DOI] [PubMed] [Google Scholar]
- 20.Bacigalupo A, Ballen K, Rizzo D, et al. . Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsson R, Remberger M, Schaffer M, et al. . Graft failure in the modern era of allogeneic hematopoietic SCT [published correction appears in Bone Marrow Transplant. 2013;48(4):616]. Bone Marrow Transplant. 2013;48(4):537-543. [DOI] [PubMed] [Google Scholar]
- 22.Przepiorka D, Weisdorf D, Martin P, et al. . 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 23.Shulman HM, Sullivan KM, Weiden PL, et al. . Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204-217. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. [Google Scholar]
- 25.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16(8):901-910. [DOI] [PubMed] [Google Scholar]
- 26.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34(2):187-220. [Google Scholar]
- 27.Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18(12):1489-1500. [DOI] [PubMed] [Google Scholar]
- 28.Armand P, Kim HT, Logan BR, et al. . Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubio MT, Savani BN, Labopin M, et al. . Impact of conditioning intensity in T-replete haplo-identical stem cell transplantation for acute leukemia: a report from the acute leukemia working party of the EBMT. J Hematol Oncol. 2016;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bashey A, Zhang MJ, McCurdy SR, et al. . Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide [published correction appears in J Clin Oncol. 2019;37(6):528]. J Clin Oncol. 2017;35(26):3002-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]