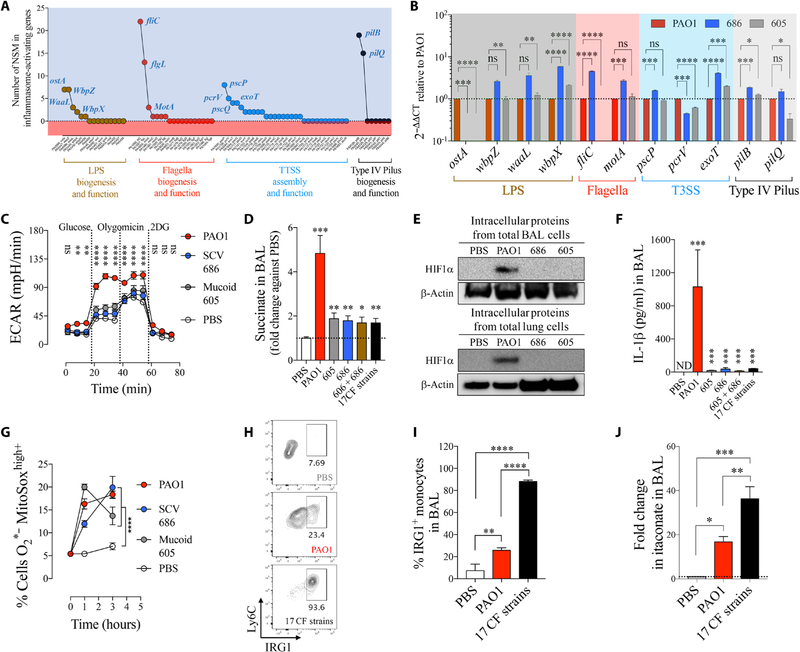
Fig. 5. Succinate-adapted P. aeruginosa isolates evoke an inefficient IRG1-itaconate–mediated immune response.
(A) Number of nonsynonymous mutations found in all P. aeruginosa isolates in PAMPs known to activate the IL-1β–HIF1α–succinate axis (inflammasome). (B) Fold mRNA expression relative to PAO1 for PAMPs with the most NSM in SCV 686 and mucoid 605 (n = 3). (C) Extracellular acidification rate (ECAR) in human monocytes treated either with PBS, PAO1, SCV 686, or mucoid 605 strain. Increased ECAR is an expected response to augmented glycolysis (n = 2). (D) Fold change in succinate in airways of WT mice treated for 24 hours with PBS, PAO1, with a single or a mixture of P. aeruginosa clinical isolates (six to seven mice per group pooled from n = 3). (E) BAL and lung HIF1α levels from total intracellular protein of mice infected as in (D). (F) IL-1β in airways of mice treated as in (D) (six to seven mice per group pooled from n = 3). (G) Mitochondrial ROS in human THP-1 cell induced by PAO1 or CF P. aeruginosa isolates (n = 3). (H and I) IRG1+ monocytes (six mice per group pooled from n = 2) and (J) itaconate in BAL of PBS-, PAO1-, or 17 CF strains–treated mice (four mice per group pooled from n = 2). Data are shown as means ± SEM. (B), (D), (F), (I), and (J) were analyzed by one-way ANOVA; and (C) and (G) were analyzed by two-way ANOVA. ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05.