Figure 3.
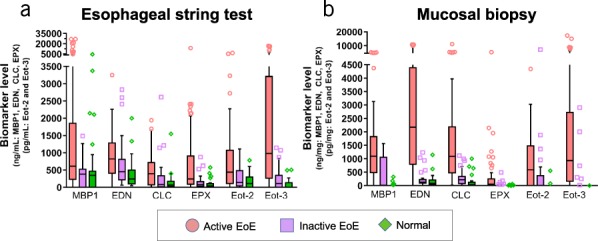
Luminal concentrations of eosinophil-associated biomarkers measured in EST samples differentiate subjects with active EoE, inactive EoE, or normal esophagus. Biomarkers were measured by ELISA in EST and mucosal biopsy samples. Levels in biopsy extracts were normalized to total protein content and are reported as ng biomarker/mg total protein. Biomarker levels in EST samples are reported as ng/mL of EST supernatant. Results are presented as box/whisker plots where the horizontal line and diamond inside the box are median and mean values, respectively; the box is the interquantiles; the lower and upper ends of whiskers are 5th and 95th percentiles, respectively; and symbols are data points with extreme values. Differences in protein biomarker levels across patient groups were compared using nonparametric ANOVA (refer to Supplemental Table S1 for P-values, see Supplementary Digital Content 1, http://links.lww.com/AJG/B281). CLC, Charcot-Leyden crystal protein; EoE, eosinophilic esophagitis; EDN, eosinophil-derived neurotoxin; Eot2, eotaxin 2; Eot3, eotaxin 3; EPX, eosinophil peroxidase; EST, esophageal string test; MBP-1, eosinophil granule major basic protein 1.
