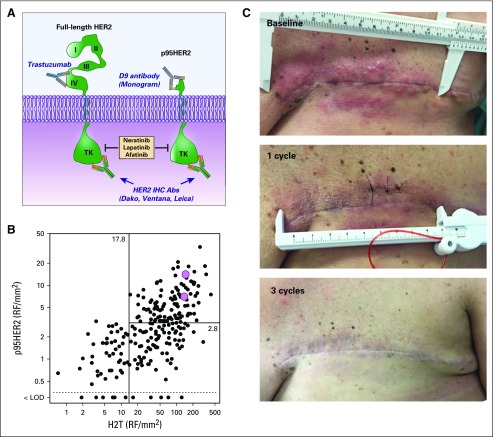
FIG 3.
Response in a patient who expressed p95HER2. (A) Anti–human epidermal growth factor receptor 2 (HER2) antibodies from Dako (Agilent, Santa Clara, CA), Ventana Medical Systems (Oro Valley, AZ), and Leica Biosystems (Wetzlar, Germany), currently used for diagnostic immunohistochemical (IHC) evaluation of breast cancer, do not distinguish between expression of full-length HER2 and the truncated p95HER2. In contrast, the antibody developed by Monogram Biosciences specifically detects the truncated p95HER2 form of the receptor and is used in the VeraTag assay. (B) Pink circles indicate the pretreatment total HER2 (H2T) and p95HER2 levels for two patients who achieved complete response (one of whom is represented in the photos in panel C) superimposed for comparison on a previously published scattergram of values obtained from a similar cohort of patients with trastuzumab-treated, metastatic HER2-positive breast cancer (LOD, p95HER2 below the limit of detection). (C) The patient initially presented with locally advanced disease with progressive chest wall disease on trastuzumab plus pertuzumab therapy. By cycle 3, the patient had complete disappearance of chest wall disease and had a clinical complete response. Abs, antibodies; LOD, logarithm of odds; RF, relative fluorescence; TK, tyrosine kinase. Panel B scattergram reproduced with permission.21