Abstract
Neuregulin (NRG), a growth and differentiation factor that signals via erbB receptor tyrosine kinases, has been shown to have biological effects in both the CNS and the peripheral nervous system. We report here that erbB4 is expressed in mature cerebellar granule cells, where it appears to be concentrated at the granule cell postsynaptic terminals. We also show that one form of NRG, Ig-NRG, plays a crucial role in aspects of cerebellar granule cell development in vitro. First, Ig-NRG treatment of granule cells in culture selectively induces the expression of the GABAA receptor β2 subunit. This increase in subunit expression is paralleled by an increase in functional GABAA receptors. In contrast to its effects on GABAA receptor subunit expression, Ig-NRG does not upregulate NMDA receptor N2B and N2C subunit expression. Second, we demonstrate that Ig-NRG also enhances neurite outgrowth from cultured granule cells. Ig-NRG does not, however, act as a survival factor for the granule cells. We have compared the effect of Ig-NRG with the effects of brain-derived neurotrophic factor (BDNF), a neurotrophin that exerts specific effects on granule cells in culture, and found that BDNF does not mimic the effects of Ig-NRG on GABAAreceptor subunit expression. Our results show that Ig-NRG has specific effects on granule cell development and maturation and may regulate these processes in vivo.
Keywords: erbB receptors, neuregulin, cerebellum, neurite outgrowth, GABAA receptors, neuronal differentiation, cerebellar granule cells
The neuregulins (NRGs) are members of the epidermal growth factor (EGF) superfamily of growth and differentiation factors (Lemke, 1996). NRG (also called NRG1), the first member of the NRG family, is also known by several other names because it was identified on the basis of different biological activities, including the activation of the HER2/erbB2/neu tyrosine kinase receptor [neu differentiation factor (Peles et al., 1992); heregulin (Holmes et al., 1992)], the induction of acetylcholine receptor expression in skeletal muscle [acetylcholine receptor-inducing activity (Falls et al., 1993)], and the induction of proliferation of Schwann cells [glial growth factor (Marchionni et al., 1993)]. NRG appears to be critical for the development of several cell types and organs, including the heart, the peripheral nervous system (PNS) and CNS (for review, see Lemke, 1996). Even though the CNS is the site of highest expression of NRG (Holmes et al., 1992) and of one of the NRG receptors, erbB4 (Plowman et al., 1993), the roles of these molecules in neuronal differentiation in the brain remain largely unknown.
One of the processes proposed to be regulated by NRG is the formation of synapses, an hypothesis that has been tested in the PNS. In particular, NRG is expressed in spinal cord motoneurons, and it increases the synthesis of nicotinic acetylcholine receptors (nAChRs) (Falls et al., 1993) and the number of Na+channels (Corfas and Fischbach, 1993) in skeletal muscle, suggesting that it is important in the formation of the neuromuscular junction. Moreover, animals with a single copy of the NRG gene are myasthenic, indicating that NRG plays a role in the regulation of muscle nAChRsin vivo (Sandrock et al., 1997). NRG is also expressed in preganglionic neurons in the spinal cord, and it has been shown to control the levels of nACh and GABAA receptors in their postsynaptic targets, the sympathetic neurons (Yang et al., 1998).
The role of NRG in regulating neuronal maturation and synapse formation in the CNS has been studied to a lesser extent. To determine the effects of NRG on CNS neurons, we tested the responses of dissociated cerebellar granule cells to NRG stimulation. We studied cerebellar granule neurons because in vivo NRG and erbB4 are expressed in a pattern consistent with these molecules being involved in interactions between granule cells and their synaptic inputs, the Golgi cells and the mossy fibers. Mature granule cells express high levels of erbB4 mRNA (Elenius et al., 1997), and these are the only neurons in the adult cerebellum that express this receptor. It has also been demonstrated that the presynaptic inputs to granule cells, the cerebellar Golgi cells and the neurons of the pontine nuclei, express NRG mRNA (Corfas et al., 1995). Moreover, NRG immunoreactivity has been shown to be concentrated at the glomeruli, the synapses formed by granule cell dendrites, pontine-derived mossy fiber terminals, and Golgi cell terminals (Sandrock et al., 1995).
We used an in vitro system of dissociated cerebellar granule cells to determine the effects of Ig-NRG, an NRG isoform that contains an immunoglobulin-like domain, on neuronal survival, neurite outgrowth, and neurotransmitter receptor expression. We found that Ig-NRG selectively upregulates the level of GABAAreceptor β2 subunit expression and enhances GABA-evoked currents in the granule cells. In contrast, Ig-NRG does not affect the expression of NMDA receptor N2B and N2C subunits (NR2B and NR2C). We found that the role of Ig-NRG in cerebellar granule cell differentiation differs from that of brain-derived neurotrophic factor (BDNF), a factor known to mediate granule cell survival (Segal et al., 1992; Lindholm et al., 1993) and neurite outgrowth (Segal et al., 1995). While both Ig-NRG and BDNF enhance neurite outgrowth from the granule cells, BDNF does not induce the expression of the GABAA receptor subunits examined in this study. In contrast to BDNF, Ig-NRG is not a survival factor for these cells. Our results suggest that, in vivo, NRG may regulate morphological and functional aspects of granule cells and their synapses and that these effects are distinct from those of BDNF.
MATERIALS AND METHODS
Recombinant NRG
The form of NRG used in these studies is the form containing an Ig-like domain [NDF β1 (14–246); Amgen, Thousand Oaks, CA]. For these studies we used a range of concentrations of Ig-NRG. TheKd for NRG binding to its receptors is ∼105 pm (Holmes et al., 1992). Thus, 1 nm NRG is a saturating concentration (Holmes et al., 1992; Dong et al., 1995), and this concentration has been shown to affect several cell types (Martinou et al., 1991; Dong et al., 1995;Canoll et al., 1996; Rio et al., 1997). In some experiments we used higher concentrations of Ig-NRG (2–5 nm), because a previous report suggested that higher concentrations of Ig-NRG may be required for certain biological effects on granule cells (Ozaki et al., 1997). The ED50 for BDNF's biological effects lies between 0.3 and 3 ng/ml. Thus in our experiments we used a saturating concentration of this factor (20–50 ng/ml), the same range of concentrations of BDNF that are usually used.
Dissociated cerebellar granule cell cultures
Cerebella were removed from postnatal day 6 (P6) Sprague Dawley rats in ice-cold PBS. The tissue was cleaned of meninges, cut into small pieces, and digested in 0.05% trypsin (Sigma, St. Louis, MO) for 15 min at 37°C. After centrifugation, the tissue was further triturated in solution containing 4000 units of DNase (Worthington, Freehold, NJ). After allowing larger, undissociated pieces of tissue to settle, the supernatant was harvested, and cells were plated on tissue culture dishes coated with 0.1 mg/ml poly-d-lysine (Collaborative Biomedical Products, Bedford, MA) and 5 μg/ml laminin (Collaborative Biomedical Products). Cells were grown in serum-free supplemented media [Neurobasal media (Life Technologies, Gaithersburg, MD), B27 supplement (Life Technologies), 6 gm/l d-glucose, 2 mml-glutamine, 100 U/ml penicillin and 100 U/ml streptomycin (pen–strep), and 25 mm KCl] (Gault and Siegel, 1997), unless noted otherwise. Granule cells were treated with Ig-NRG (1–5 nm) or BDNF (20 or 50 ng/ml; Amgen) at the time of plating. In experiments in which granule cells were cultured for >2 d, cells were treated with 60 μm5-fluoro-2′-deoxyuridine at 2 d in vitro (DIV) to inhibit proliferation of non-neuronal cells.
Granule cell reaggregate cultures
Reaggregate cultures of granule cells were obtained using the method of Segal et al. (1995) with slight modifications. Granule cells were prepared as described above. Dissociated cells were incubated for 24 hr on uncoated tissue culture dishes in media containing DMEM, 10% fetal bovine serum, d-glucose, pen–strep, and glutamine. Then, reaggregates were washed in serum-free media [Neurobasal media, N2 supplement (Life Technologies), glucose, pen–strep, and glutamine] and plated in this media onto dishes coated with 500 μg/ml poly-d-lysine. At the time of replating, the reaggregates were treated with 1 nm Ig-NRG, 5 nm Ig-NRG, or 20 ng/ml BDNF. After 22 hr, the reaggregates were fixed and stained with a class III β-tubulin antibody (1:1000; Sigma) and a Cy3-conjugated secondary antibody (1:1000; Jackson ImmunoResearch, West Grove, PA) to visualize the neurites, and neurite length was analyzed.
Reaggregates consist of a cluster of cell bodies surrounded by a halo of neurites. To analyze neurite length, the reaggregates were visually divided into quadrants, and the length of the longest neurite in each quadrant was measured from the edge of the cell body cluster. The four quadrant lengths for each reaggregate were averaged to obtain the neurite length value for each reaggregate. Only reaggregates with a cell body cluster between 50 and 150 μm were included in the analysis. For each group (control, 1 nm Ig-NRG, 5 nm Ig-NRG, or BDNF) 36 reaggregates were measured in three experiments.
Survival assays
MTT assay. Dissociated granule cells were plated in 96-well plates at a density of 6 × 104 cells/well. At 4 DIV cells were washed twice and cultured for 2 d in minimal media (BME + 0.2% BSA) with the following treatments: 1 nm Ig-NRG, 5 nmIg-NRG, 20 ng/ml BDNF, or 10 μg/ml insulin. At 2 d after the media change, MTT (0.7 mg/ml; Sigma) was added, and cells were incubated at 37°C for 2 hr. Solubilization buffer (50% dimethylformamide, 10% SDS, and 20% glacial acetic acid) was added, and the cells were kept overnight at 37°C in a humidified chamber. The next day an OD of the wells was read at 595 nm. In parallel experiments the linearity of the assay was tested by measuring OD in cultures containing different numbers of NIH 3T3 cells. We found that the assay was linear within the OD range used in all our experiments.
Hoechst assay. The cells were cultured and treated in the same conditions used for the MTT assay, except that 1 d after the addition of the trophic factors, the cells were fixed and stained with Hoechst 33342 (Molecular Probes, Eugene, OR). The percent of apoptotic cells was then quantified in the cultures.
Immunocytochemistry of cultured granule cells
Granule cells in 24-well plates (35–40 × 104 cells/well) were fixed in 4% paraformaldehyde in PBS for 15–20 min at room temperature. Cells were washed in PBS and then blocked and stained using conditions appropriate for each antibody (see below).
Phospho-CREB. Granule cells were initially plated in serum-containing media. At 2 DIV, the cells were serum starved for 5 hr and then treated with 1 nm Ig-NRG for 20 min. Cells were immediately fixed, permeabilized with PBS and 0.5% NP-40 for 10 min, washed again, blocked in 3% BSA for 1 hr, and incubated with a phospho-CREB antibody (1:1000) (Ginty et al., 1993) overnight at 4°C. Cells were then washed and incubated with an anti-rabbit biotinylated secondary antibody (1:1000; Vector Laboratories, Burlingame, CA), and the reaction product was visualized by diaminobenzidine reaction.
ErbB4 staining. Cells were blocked in 3% BSA and 0.1% Triton X-100 in PBS (blocking buffer) for 1 hr at room temperature and incubated overnight at 4°C with an anti-erbB4 antibody (C-18; 1:200; Santa Cruz Biotechnology, Santa Cruz, CA). Cells were washed with PBS, incubated with a Cy3 anti-rabbit secondary antibody (1:1000 in blocking buffer; Jackson ImmunoResearch) for 1 hr at room temperature, washed again, and mounted.
Immunocytochemistry of adult rat cerebellum
Adult rats were anesthetized with CO2 and fixed by cardiac perfusion with 4% paraformaldehyde in PBS. Cryosections (20 μm) were blocked (3% BSA and 0.1% Triton X-100 in PBS) for 1 hr at room temperature, followed by incubation with polyclonal anti-erbB4 0615 or 0618 (Zhu et al., 1995) (1:200) and monoclonal anti-synaptophysin (1:20; Boehringer Mannheim, Indianapolis, IN) antibodies at 4°C overnight. The sections were washed with PBS, and the detection procedure was performed using an anti-rabbit Cy3 secondary antibody (Jackson ImmunoResearch) and an anti-mouse Oregon green secondary antibody (Molecular Probes) (each at 1:1000) for 1 hr at room temperature. Slides were mounted with Gel/Mount (Biomeda Corporation, Foster City, CA), and staining was visualized by light or confocal microscopy. Images were captured using a digital camera (Orca; Hamamatsu) in a fluorescence microscope or by confocal microscopy (OZ confocal microscope; Odyssey, Noran Instruments, Middleton, WI).
Western blot analysis
Phosphotyrosine Western blots were performed on whole-cell lysates of granule cells. Granule cells (2 DIV) were treated with 1 nm Ig-NRG or vehicle (PBS) for 5 min. Cells were lysed with 2× DTT sample buffer (125 mm Tris-HCl, pH 6.8, 20% glycerol, 6% SDS, 0.1 mg/ml bromophenol blue, and 100 mmdithiothreitol). For the GABAA receptor β2/3 Western blot, crude membranes were prepared from granule cells. Cells were harvested in Tris-buffered saline and centrifuged at 1000 rpm for 7 min at 4°C. The cells were homogenized with 0.5 ml of homogenization buffer containing 25 mm Tris-HCl, pH 7.4, 5 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 20 mg/ml leupeptin, 0.1% aprotinin, 1 mmiodoacetamide, 200 mg/ml bacitracin, and 20 mg/ml soybean trypsin inhibitor for 10 min on ice and then sonicated for 15–20 sec. The cells were then centrifuged at 10,000 rpm for 30 min at 4°C, and the pellet was resuspended in 20–25 ml of homogenization buffer. The protein was quantified using the BCA method according to the manufacturer's protocol (Pierce, Rockford, IL).
Aliquots of whole-cell lysates or membrane preparations were resolved on SDS polyacrylamide gel electrophoresis and transferred to Immobilon-P (polyvinylidene difluoride) membranes (Millipore, Bedford, MA). The membranes were blocked and incubated with either a mouse monoclonal anti-phosphotyrosine antibody (4G10; 1:10,000) followed by a peroxidase-conjugated anti-mouse secondary antibody (1:20,000; Boehringer Mannheim) or with an antibody that recognizes both the β2 and β3 (for the β2/3) subunits (bd17; Boehringer Mannheim; 1:20) followed by peroxidase-conjugated goat anti-mouse IgG (1:1000; Kirkegaard & Perry, Gaithersburg, MD). Blots were developed with Renaissance (DuPont NEN, Boston, MA) and exposed to autoradiography film (Reflection; DuPont NEN).
Reverse transcription-PCR
Comparative reverse transcription (RT)-PCR was performed as described previously (Behringer et al., 1996), with slight modifications. RNA was prepared from dissociated granule cells (2 DIV; plated at a density of 3.1 × 106cells/35 mm dish) using the Ultraspec RNA Isolation Reagent (Biotecx Laboratories, Houston, TX). RNA (0.2 μg) was treated with DNase (Boehringer Mannheim) and then transcribed to cDNA using Superscript RT (Life Technologies). The PCR reaction was performed in buffer containing [32P]dCTP and primers specific for neurotransmitter receptors (GABAAreceptors or NMDA receptors). In addition, a separate reaction was performed in parallel for each sample, using primers specific for elongation factor 1-α (EF1-α), a transcript that remains at a constant level over time in cultured granule cells. Each primer set was first tested for the linearity of the PCR amplification before the experiments. PCR reactions with all GABA receptor subunit primers were linear between 20 and 50 cycles, and therefore 31 cycles were used in all reactions. For the NMDA receptor subunits, reactions were linear between 30 and 50 cycles, and therefore 36 cycles were used for quantification. In each case primers for EF1-α, which behaved linearly between 20 and 50 cycles, were used with the same number of cycles as that for the specific receptor subunit tested. All reactions were run on the PTC-1000 Thermal Controller (MJ Research, Watertown, MA) for the appropriate number of cycles: 92°C for 1 min, 55°C for 30 sec, and 72°C for 45 sec. PCR products were separated on 8% nondenaturing polyacrylamide gels. The gels were dried and exposed to the Molecular Dynamics Phosphor Screen (Sunnyvale, CA), and the intensity of the bands was quantified using IPLab Gel software. The intensity of the bands corresponding to the neurotransmitter receptor subunits was normalized relative to the intensity of the EF1-α band in each sample and then compared among the different treatments.
Another RT-PCR protocol was also used to confirm the observed changes in gene expression, and similar results were obtained. Briefly, 83 pg of RNA transcribed from the bacterial plasmid SP64 (Promega, Madison, WI) was added to each sample of granule cell RNA to control for variability between samples. Then, primers for the gene of interest and for SP64 were used in the same reaction, and the PCR products were separated by gel electrophoresis. Each band was excised and measured by scintillation counting. The level of labeling in the bands corresponding to the neurotransmitter receptors was normalized relative to the levels of SP64 PCR product and then compared among the different treatments. Each RT-PCR reaction for every RNA sample was performed at least twice.
The following primer sequences for PCR were used [(+) is complementary to the noncoding strand, and (−) is complementary to the coding strand]: GABAA receptor subunits, α1 (+) = 1410–1429 and α1 (−) = 1501–1520 (Khrestchatisky et al., 1989), β2 (+) = 1808–1831 and β2 (−) = 1882–1905 (Ymer et al., 1989), and γ2 (+) = 1744–1768 and γ2 (−) = 1807–1829 (Shivers et al., 1989); NMDA receptor subunits, NR2B (+) = 4015–4034 and NR2B (−) = 4217–4236 (Yoshioka et al., 1996) and NR2C (+) = 845–864 and NR2C (−) = 1029–1048 (Yoshioka et al., 1996); EF1-α (+) = 313–337 and EF1-α (−) = 462–485 (Sundstrom et al., 1990); and SP64 (+) = 234–254 and SP64 (−) = 344–364 (Beattie and Siegel, 1993).
Whole-cell patch-clamp electrophysiology and focal application of drugs
Coverslips of cultured granule cells were affixed to a 35 mm culture dish and bathed in an external solution containing (in mm): 137 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, and 5 HEPES, pH 7.4. Recordings were made at room temperature (22–24°C). Cells were visualized using an inverted microscope equipped with Hoffman modulation-contrast optics. Patch-clamp recording pipets were fabricated from borosilicate glass capillary tubing with an internal filament (1.5 mm outer diameter; Sutter Instruments, Novato, CA). The recording pipets were fire polished to resistances ranging between 8 and 10 MΩ using a Flaming Brown micropipet puller (Sutter Instruments) and filled with a recording solution containing (in mm): 140 KCl, 1.8 CaCl2, 1.0 MgCl2, and 5 HEPES, pH 7.4. Mg2+-ATP (1 mm) was added to the recording solution to prevent possible rundown of agonist-induced currents. Cells were voltage clamped at −60 mV. Compensation for series resistance and liquid junction potentials was routinely applied. Whole-cell GABA-induced currents were recorded with an EPC-7 patch-clamp amplifier (Heka Electronics, Lambrecht, Germany). Data were acquired, digitized, and analyzed using DATAQ/DATANAL software (JPM Programming). Peak amplitudes of GABA-activated current responses from each cell were derived by determining the maximal amplitude of current responses elicited by a saturating concentration of GABA (100 or 125 μm).
Focal application of drugs was as follows: GABA was prepared from frozen stock and diluted in bath solution immediately before each experiment. An eight-barrel drug pipet assembly was used to apply drugs. Seven barrels were filled with varying concentrations of GABA (0.1–150 μm). The eighth barrel was routinely filled with recording solution that was applied continuously between epochs of GABA application to clear the agonist from the immediate vicinity of the cell and to control for possible mechanical artifacts attributable to bulk flow. The multibarrel assembly was mounted on a micromanipulator, and the tip of the multibarrel drug pipet assembly was navigated under microscopic control to within 2–4 μm of the cell under study. GABA was applied via regulated pressure (<3 psi) using a Picospritzer (General Valve, Fairfield, NJ) driven by a set of pulse generators (A310 Accupulser; WPI). The concentrations of drugs are those used to fill the drug barrels and represent the maximal limit to which a cell would be exposed.
Statistical analysis
Statistical analyses were performed using StatView software. For survival data and reaggregate data, ANOVA and Fisher's LSD tests were performed. For single-cell neurite outgrowth data, unpairedt tests were performed. GABAA and NMDA receptor mRNA data were analyzed by the nonparametric Wilcoxon signed rank test. Statistical significance was determined at thep < 0.05 level.
RESULTS
erbB4 receptors are concentrated at glomerular synapses in the mature cerebellum
We have shown previously by in situ hybridization that erbB4 mRNA is highly expressed in the granule cell layer of adult mice (Elenius et al., 1997). To determine the subcellular localization of erbB4, we stained sections of adult rat cerebellum with anti-erbB4 antibodies. We found that erbB4 immunoreactivity in the granule cell layer was closely associated with the synaptic vesicle protein synaptophysin (Fig. 1), suggesting that it is localized to the glomerular synapses. Because erbB4 is expressed by the granule cells (Elenius et al., 1997), it is most likely that this receptor is localized to their postsynaptic terminals. It has been shown previously that the granule cell afferents, the cerebellar Golgi cells and the neurons in the pontine nucleus, express NRG (Corfas et al., 1995) and that NRG protein is concentrated at the glomeruli (Sandrock et al., 1995). Thus, it is likely that the erbB4 receptors present in the postsynaptic side and the NRG released by the presynaptic terminals mediate interactions between the granule cell neurons and their presynaptic inputs. On the basis of these findings we set out to study the effects of NRG on cerebellar granule cellsin vitro.
Fig. 1.
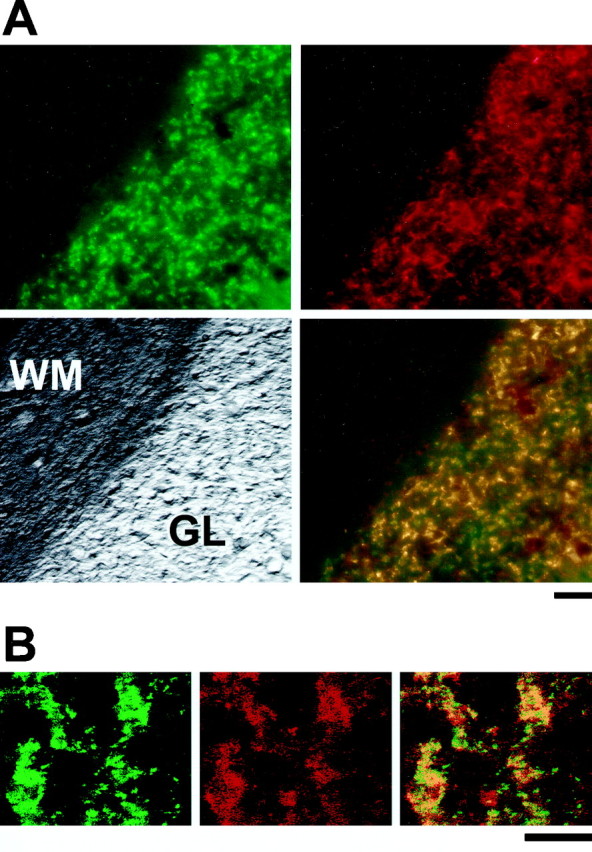
erbB4 and synaptophysin immunoreactivity colocalize at cerebellar glomeruli. A,Top, Parasagittal sections of adult cerebellum were stained with a monoclonal antibody to the synaptic vesicle protein synaptophysin (Oregon green secondary antibody; left) and polyclonal antibodies to erbB4 (0618) and visualized with a Cy3 secondary antibody (right). Bottom, The two top images are superimposed (right), and a bright-field image of the section is shown (left).B,Left, Middle, Confocal images were obtained at higher magnification of the granule cell layer from adult cerebellum stained as described above (synaptophysin,green; erbB4, red). Right, Colocalization of the two proteins is shown by the superimposition of the two images. GL, Granule cell layer; WM, white matter. Scale bars: A, 20 μm; B, 50 μm.
Granule cells in culture respond to Ig-NRG
The NRG gene, via alternative splicing, produces many different NRG isoforms (Lemke, 1996). In all of the experiments included in this study, an isoform of NRG that contains an immunoglobulin-like domain located N terminal to the EGF-like domain (Ig-NRG = NDFβ114–246) was used. This is the isoform expressed in the cerebellar Golgi cells and the neurons of the pontine nucleus (Corfas et al., 1995) and therefore is the isoform that granule cells are most likely exposed to in vivo.
We used cultures of granule cells obtained from P6 rat cerebella, which are highly enriched in granule neurons derived from the external granule cell layer (EGL) (Raetzman and Siegel, 1999). Although the EGL granule cells in vivo express NRG, its expression is rapidly downregulated as the cells mature in culture (Rio et al., 1997). We now show that, accompanying the decrease in NRG expression, granule cells express erbB4 by 2 d in vitro (Fig.2A). These changes in the pattern of expression of NRG and erbB4 in culture mirror those observed as granule cells mature in vivo.
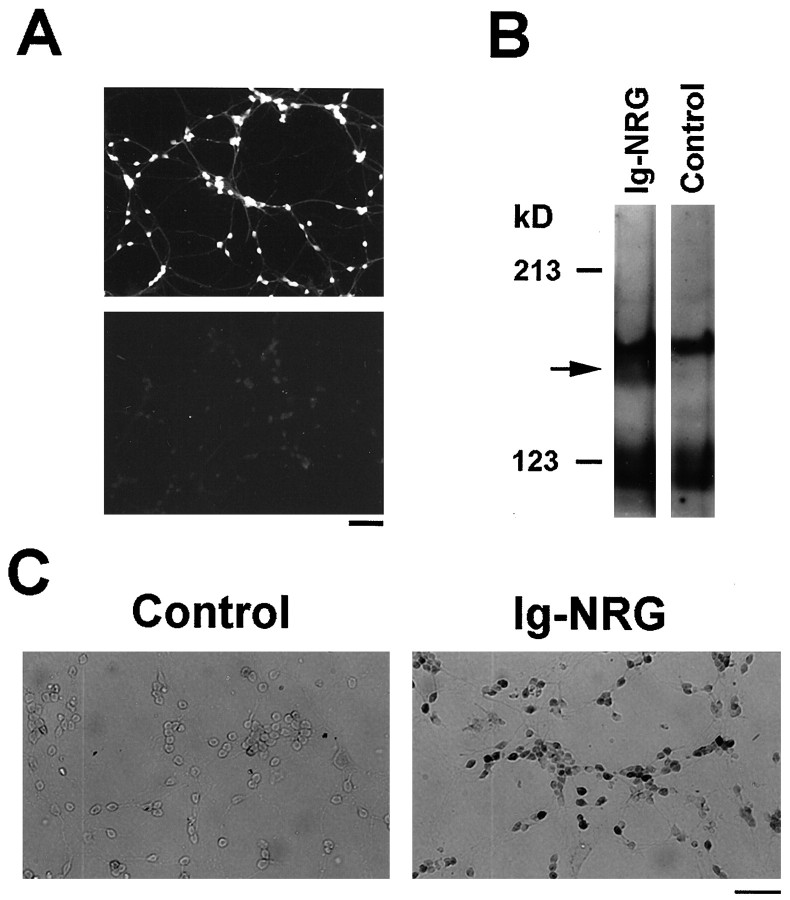
Fig. 2.
Granule cells in culture express erbB4 and respond to Ig-NRG. A, Granule cells express the erbB4 receptor.Top, Granule cells at 2 DIV were stained with a polyclonal anti-erbB4 antibody and a Cy3 secondary antibody. Immunoreactivity was present in the cell bodies and processes of the cells. Bottom, No staining was observed when the erbB4 antibody was preincubated with a cognate peptide.B, Ig-NRG induces p180 tyrosine phosphorylation in granule cells. Granule cells at 2 DIV were stimulated with 1 nm Ig-NRG or vehicle (control) for 5 min. Lysates were resolved by 5% SDS-PAGE, and the blot was probed with an anti-phosphotyrosine antibody. The Ig-NRG-induced tyrosine-phosphorylated band is indicated by the arrow.C, Ig-NRG induces CREB phosphorylation in cultured granule cells. Serum-starved granule cells were treated with Ig-NRG (1 nm for 20 min), fixed, and stained with a phospho-CREB antibody followed by peroxidase reaction. There were significantly more labeled cells in the Ig-NRG-treated cultures (cells appeardark). Scale bars: A, 40 μm;C, 50 μm.
Expression of erbB4 in the granule cells suggested that they should respond to NRG. We found that treatment of cultured granule cells with 1 nm Ig-NRG for 5 min led to a significant and reproducible tyrosine phosphorylation of a 180 kDa protein (Fig.2B). This band has the molecular weight of the erbB receptors and is similar to that induced by NRG stimulation in muscle cells (Corfas et al., 1993), astrocytes (Rio et al., 1997), and neurons (Corfas et al., 1995). These results show that granule cells respond to Ig-NRG by activation of erbB receptors.
We have shown previously that the EGF-like domain of NRG can induce phosphorylation of the transcription factor CREB in cultured cerebellar astroglia (Rio et al., 1997), and similar results have been demonstrated in Schwann cells in vitro (Taberno et al., 1998). To test whether NRG induced CREB phosphorylation in cultured granule cells, we stained untreated and Ig-NRG-treated cells with an antibody that recognizes CREB phosphorylated in Ser133 (Ginty et al., 1993). Treatment with Ig-NRG led to more than a threefold increase in the number of cells with phospho-CREB-positive nuclei (18.3 ± 11.4% labeled cells in control; 60.5 ± 3.9% in Ig-NRG-treated cells) (Fig. 2C). These results suggest that granule cells can respond to Ig-NRG by activation of erbB receptors, which then leads to activation of transcription factors that may regulate gene expression in these neurons.
Ig-NRG does not support granule cell survival
Several growth factors that signal through tyrosine kinase receptors have been shown to act as survival and differentiation factors for cerebellar granule cells (Lindholm et al., 1997). We investigated the biological effects of NRG on the granule cells and tested the specificity of these effects by comparing them with the effects of BDNF, which supports granule cell survival (Segal et al., 1992; Lindholm et al., 1993) and induces neurite outgrowth from granule cells (Segal et al., 1995).
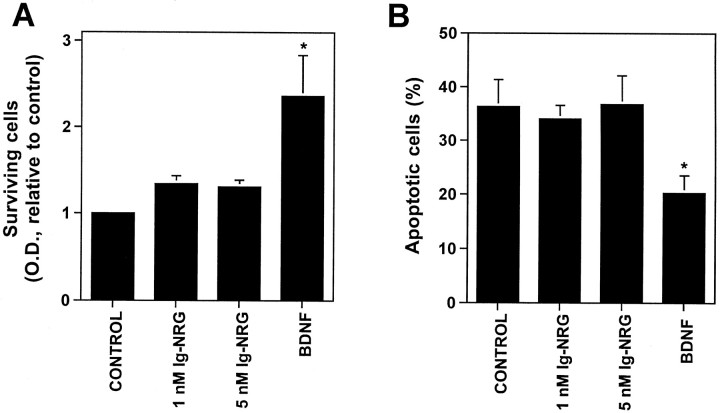
We first compared the effects of Ig-NRG with the effects of BDNF on granule cell survival using two assays, an MTT assay to measure surviving cells and Hoechst nuclear staining to quantify the number of apoptotic cells. By the MTT assay we found that in the absence of any other factors, BDNF increased the survival of granule cells in culture by more than twofold (p < 0.0001) (Fig.3A). In contrast, Ig-NRG had no effect on granule cell survival (1 nm,p = 0.10; 5 nm,p= 0.15) (Fig. 3A). Quantification of the percent of apoptotic cells in the cultures showed similar results. Granule cells were incubated under the different conditions for 24 hr, fixed, and stained with the nuclear dye Hoechst, and apoptotic cells were recognized by the condensation of their nuclei. Only cultures treated with BDNF had significantly less apoptotic cells (p = 0.014) than did control (Fig.3B). These results show that Ig-NRG does not support granule cell survival in vitro.
Fig. 3.
Ig-NRG is not a survival factor for granule cells. A, Granule cells in minimal medium were treated with 1 nm Ig-NRG, 5 nm Ig-NRG, 20 ng/ml BDNF, or vehicle (control) for 2 d. Cell survival was then measured by the MTT assay, and the reaction product was analyzed spectrophotometrically. The graph shows an average OD for four independent experiments. Cell survival was significantly increased only by BDNF treatment (BDNF, *p < 0.0001; 1 nm NRG, p = 0.10; 5 nm NRG,p = 0.15). B, Granule cells were treated with 1 nm Ig-NRG, 5 nm Ig-NRG, 20 ng/ml BDNF, or vehicle (control) for 24 hr. Cells were then fixed and stained with Hoechst dye, and the percentage of apoptotic cells was quantified. BDNF but not Ig-NRG treatment significantly reduced the percent of apoptotic cells compared with control (BDNF, *p = 0.014; 1 nm Ig-NRG, p = 0.71; 5 nm Ig-NRG, p = 0.93).
Ig-NRG induces neurite outgrowth
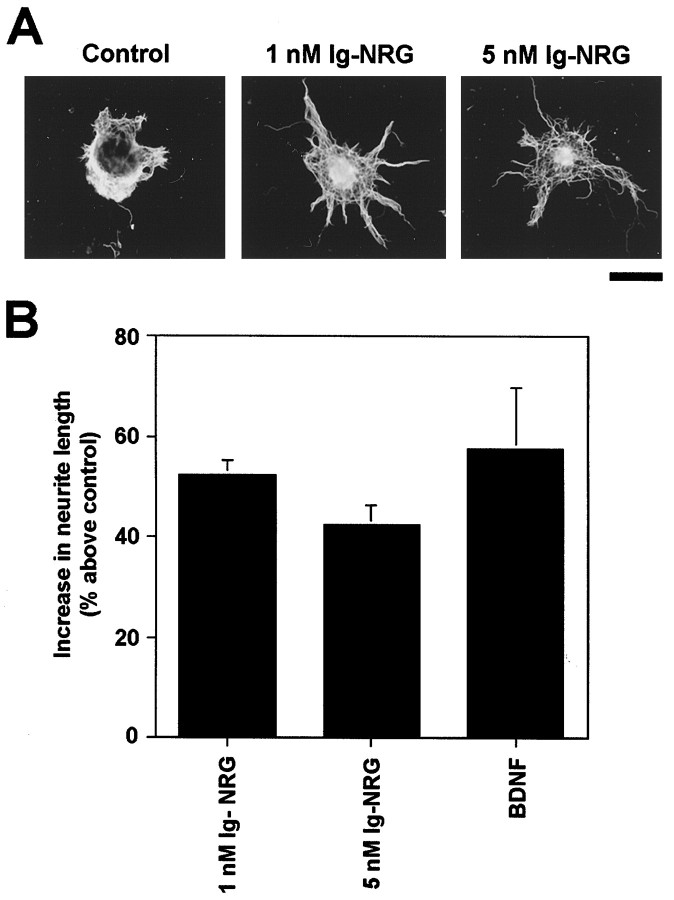
BDNF has also been shown to induce neurite outgrowth from granule cells in reaggregate cultures (Segal et al., 1995). We used a similar culture system to study the effects of Ig-NRG on neurite outgrowth. Granule cell reaggregates were treated for 22 hr with vehicle (control), 1 nm Ig-NRG, 5 nm Ig-NRG, or 20 ng/ml BDNF. Cells were then fixed and stained with antibodies against class III β-tubulin antibody to visualize the neurites, and neurite length was measured. We found that neurites from granule cell reaggregates treated with Ig-NRG or BDNF were significantly longer than those from untreated reaggregates (p < 0.0001 for all treatments) (Fig. 4). Other parameters of neurite outgrowth including thickness of neurite bundles and neurite fasciculation were not qualitatively different among the treatments. To examine further the effect of Ig-NRG on neurite outgrowth, we tested its effect on dissociated granule cells. As in the reaggregates, Ig-NRG treatment caused a significant increase (45% above control) in the neurite length of dissociated granule cells (control, 52.3 ± 5.6 μm average length; 2 nm Ig-NRG, 75.7 ± 7.1 μm average length;p = 0.01).
Fig. 4.
Ig-NRG enhances neurite outgrowth from granule cell reaggregates. A, Granule cell reaggregates were treated with vehicle (control), 1 nm Ig-NRG, or 5 nm Ig-NRG for 22 hr. Cultures were then fixed and stained with an antibody to class III β-tubulin and visualized with a Cy3 secondary antibody. Scale bar, 100 μm. B, Ig-NRG and BDNF significantly increase neurite outgrowth from granule cell reaggregates. Reaggregates were treated with 1 nm Ig-NRG, 5 nm Ig-NRG, or 20 ng/ml BDNF and stained with a class III β-tubulin antibody, and neurite length was analyzed. The graph shows a percentage increase in neurite length above control (p < 0.0001 for all three treatments).
Ig-NRG treatment increases GABAA receptor β2 subunit expression in cultured granule cells
It has been shown that NRG plays an important role in the regulation of neurotransmitter receptor expression in skeletal muscle (Harris et al., 1988; Martinou et al., 1991; Falls et al., 1993) and in sympathetic neurons (Yang et al., 1998). It has also been reported that NRG regulates the expression of the NR2C subunit in cerebellar slices in culture (Ozaki et al., 1997). Our finding that Ig-NRG induces CREB phosphorylation in cerebellar granule cells suggested that Ig-NRG may activate gene transcription in these neurons. In addition, because both NRG and erbB4 are concentrated at the cerebellar granule cell glomeruli, we hypothesized that NRG might regulate the expression of two neurotransmitter receptors that function at this synapse, GABAA and NMDA receptors (Watanabe et al., 1994;Wisden, 1995).
Previous studies have demonstrated that several GABAA receptor subunit mRNAs are expressed in the cerebellar granule neurons in vivo (Laurie et al., 1992a;Persohn et al., 1992) and in culture (Bovolin et al., 1992; Behringer et al., 1996). Although the number and stoichiometry of GABAA receptor complexes in these neurons are unknown, α1, β2, and γ2 subunits are thought to contribute to an abundant receptor type (Benke et al., 1991). In addition, the expression of these subunit mRNAs is developmentally regulated in vivo, being expressed at low levels early in development and then rising during the second postnatal week, the onset of extensive synapse formation (Laurie et al., 1992b). In contrast, the levels of these subunit mRNAs remain low and constant in granule cell cultures prepared at P6 and maintained for at least 10 d in culture (Behringer et al., 1996). This suggests that the expression of these subunit transcripts requires signals present in vivo that are absentin vitro. We therefore focused on the effects of Ig-NRG on these three subunits using comparative RT-PCR (see Materials and Methods).
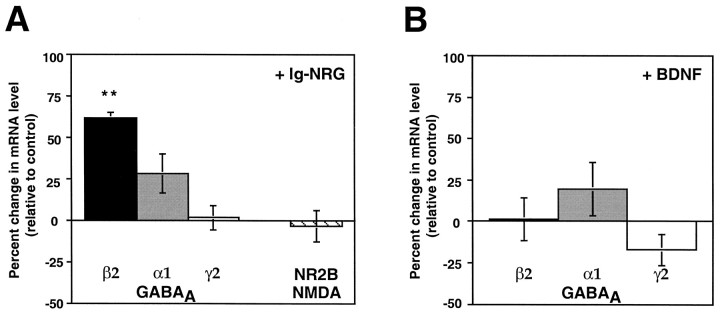
Analysis of GABAA receptor subunit mRNAs in the cultured granule cells showed that Ig-NRG (1 nm, for 2 d) specifically increases expression of the β2 subunit transcript [162% of control (p = 0.02)] (Fig.5A). In contrast, there were no significant increases in the levels of α1 or γ2 subunit mRNAs in response to Ig-NRG [α1, 128% of control (p = 0.14); γ2, 102% of control (p = 0.96)] (Fig.5A). Analysis of subunit levels after treatment with 50 ng/ml BDNF showed that BDNF did not alter the levels of any of these GABAA receptor subunit mRNAs (Fig.5B). Similar results were found with 20 ng/ml BDNF (data not shown).
Fig. 5.
Ig-NRG treatment upregulates GABAAreceptor β2 subunit mRNA expression. A,GABAA subunit mRNA levels were examined in control and Ig-NRG-treated granule cells (2 DIV). The graph shows an average percentage of change in mRNA level relative to control for five independent experiments. Although levels of GABAA β2 mRNA increased significantly in response to Ig-NRG (**p= 0.02), there was no significant effect on the levels of GABAA α1 (p = 0.14), GABAA γ2 (p = 0.96), or NMDA NR2B (p = 0.50) subunit mRNAs.B, BDNF (50 ng/ml) does not induce an upregulation of mRNA for β2 (p = 0.35), α1 (p = 0.50), or γ2 (p = 0.14) GABAA subunits. The data represent an average of three independent experiments.
The expression of NMDA receptor subunits in granule cells is also developmentally regulated. Premigratory granule cells in the EGL express NR1, NR2A, and NR2B subunits. After migration to the internal granule cell layer (IGL), when the synaptic connections onto granule cells are being formed, NR2B expression is downregulated, and the granule cells begin to express NR2C (Watanabe et al., 1992). Moreover, it has been reported that Ig-NRG treatment of cerebellar slices leads to increases in the level of mRNA for the NR2C subunit (Ozaki et al., 1997). Therefore, we tested whether Ig-NRG had any effects on NMDA receptor subunit mRNA expression in dissociated granule cells. We found that granule cells in culture express NR2B and that treatment with Ig-NRG (1 nm) for 2 DIV did not alter expression of this subunit (Fig. 5A). NR2C mRNA was expressed in both untreated and Ig-NRG-treated cultures but at levels too low to be quantified.
Having observed a change in GABAA receptor subunit mRNA, we investigated whether the level of β subunit protein was increased in response to Ig-NRG using an antibody that recognizes both the GABAA β2 and β3 subunits (Benke et al., 1991; Ewert et al., 1992). Dissociated granule cells were treated with Ig-NRG for 3 DIV. The length of the treatment was longer than in the experiments for detection of mRNA to allow for a delay between the increase in mRNA level and in protein level. After treatment, cell membranes were isolated, and the levels of GABAAβ2/3 subunit expression in Ig-NRG-treated and control cells were analyzed by Western blot. We found an increase in GABAA β2/3 signal in the treated cells (140% of control in two experiments) (data not shown). These results suggest that Ig-NRG treatment leads to an increase in the levels of GABAA β2/3 subunit protein.
Ig-NRG upregulates functional GABAA receptors in cultured granule cells
The β subunits of the GABAA receptor have been reported to play a role in receptor assembly and targeting to the cell membrane (Connolly et al., 1996; Connor et al., 1998; Luo et al., 1998). Our observation that expression of the β2 subunit is upregulated by Ig-NRG suggests that GABAAreceptor assembly and/or targeting may be enhanced by Ig-NRG treatment. Such a change would result in an increase in GABAA receptors in individual granule cells that, in turn, would be reflected in an increased maximal response to GABA. To test this possibility, current responses to varying concentrations of GABA (0.1-150 μm) were recorded from Ig-NRG-treated and control granule cells under whole-cell patch-clamp conditions. Cultures were examined between 3 and 4 DIV, when Western blot revealed an upregulation of the β2/3 GABAAreceptor subunit protein in the Ig-NRG-treated cultures. Treatment with Ig-NRG resulted in a dramatic difference in the GABA response profile, with a significant increase (greater than twofold) in the efficacy of the response (Fig. 6A). However, this was not associated with a marked change in the potency of the response to GABA. When comparing the maximal response to saturating GABA concentrations (125 or 150 μm), we found that the amplitude of the response was significantly greater in the Ig-NRG-treated granule cells than in control cells (Ig-NRG, 0.34 ± 0.05 nA; control, 0.16 ± 0.03 nA; p < 0.005) (Fig. 6B).
Fig. 6.
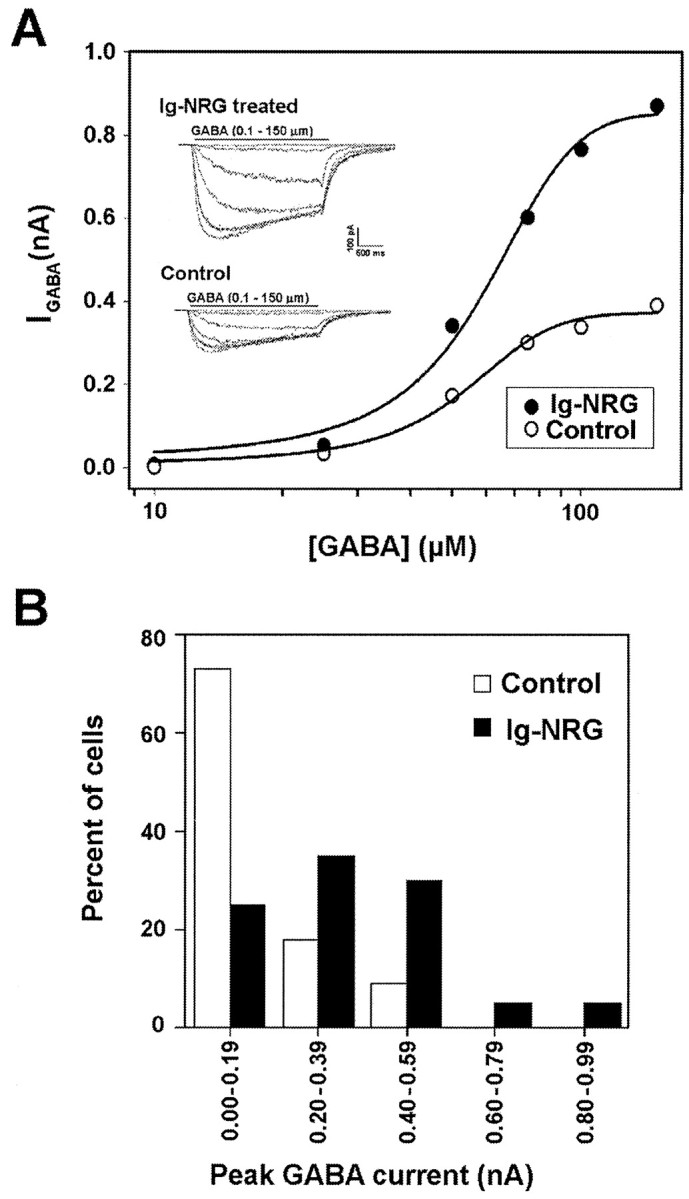
Ig-NRG increases functional GABAAreceptors in cultured granule cells. A, Representative whole-cell current responses to varying concentrations of GABA (10, 25, 50, 75, 100, and 150 μm) in a granule cell that was treated with Ig-NRG (filledcircles) and in a control cell (opencircles). Ig-NRG (3 nm) was added to the culture medium at the time of plating, and the GABA responses were assayed on 3 and 4 DIV. Inset, Superimposed computer-generated current traces recorded from the same cells (top, Ig-NRG-treated cell; bottom,control cell) used to generate the dose–response curves.B, Histogram representing the binned distribution of the amplitude of maximal current response to GABA (125 or 150 μm) recorded in granule cells from Ig-NRG-treated (n = 20; solidbars) and control (n = 22; openbars) granule cells. A greater percentage of cells examined in the Ig-NRG-treated cultures are found in the bins representing higher maximal current response amplitudes, and the mean response amplitude is significantly higher than that in control cells (Ig-NRG, 0.34 ± 0.05 nA; control, 0.16 ± 0.03 nA;p < 0.005).
DISCUSSION
NRG has different functions during early and late postnatal cerebellar development
Cerebellar granule cells undergo a well defined pattern of postnatal development. During the first 2 postnatal weeks in the rodent, granule cell precursors proliferate in the EGL. When these cells become postmitotic, they leave the EGL and migrate along the radial fibers of the Bergmann glia to their final positions in the IGL. After they reach the IGL, they form synapses with their inputs, the terminals of the mossy fibers and the cerebellar Golgi cells.
Our findings that the pattern of expression of NRG and erbB4 changes dramatically during cerebellar postnatal development (Rio et al., 1997; this study) suggest that NRG plays different roles throughout granule cell development, mediating events in early and late postnatal cerebellar development. Our previous study showed that premigratory and migrating cerebellar granule cells express NRG and that Bergmann glia express erbB4 only during the period of granule cell migration (Rio et al., 1997). We also demonstrated that erbB receptor signaling in the glial cells in vitro is crucial for radial glial process formation and for granule neuron migration (Rio et al., 1997). The present study shows that the pattern of expression of NRG and erbB4 in the adult cerebellum is very different. In the adult cerebellum erbB4 is not expressed by Bergmann glia but by the granule cells (see alsoElenius et al., 1997). At this stage NRG is not expressed by granule cells but by cerebellar Golgi cells and the pontine neurons (Corfas et al., 1995), the neurons that synapse onto the granule cells. Moreover both NRG (Sandrock et al., 1995) and erbB4 appear to be concentrated at the glomeruli, the synapses between granule cells and its presynaptic inputs (this study). Thus, these results suggest that NRG and erbB4 may mediate interactions between granule neurons and their synaptic inputs, including some that may be important for granule cell development and synapse formation.
Role of NRG in neurotransmitter receptor expression in granule cells: GABAA receptors
The level of several GABAA receptor subunit mRNAs increases in granule cells after their migration, when they have reached the IGL and are establishing synaptic connections with Golgi cell axons and mossy fiber afferents (Laurie et al., 1992a). In contrast, the levels of expression of these subunit mRNAs remain constant for up to 10 d if granule cells are isolated from P6 rats and grown in culture (Behringer et al., 1996). This disparity suggests that factors present in the intact cerebellum play a role in mediating the increases in GABAA receptor subunit expression. We now show that Ig-NRG causes an upregulation of GABAA receptor β2 subunit expression in cultured granule cells. Among the subunits we tested, the effect of Ig-NRG on GABAA receptors was specific to the β2 subunit. Ig-NRG treatment also results in an increase in peak GABA-activated whole-cell currents, indicating that Ig-NRG regulates expression of functional GABAA receptors in these cells. It has been suggested that the β subunits are critical for GABAA receptor targeting to the cell surface (Connolly et al., 1996; Connor et al., 1998; Luo et al., 1998). Thus, by regulating the expression of the β2 subunit, NRG signaling may control the total number of functional GABAAreceptors at the postsynaptic terminal of granule cells.
The effects of Ig-NRG on GABAA receptor expression in granule cells are similar to the effects of NRG on nAChR expression in skeletal muscle. In cultured chick myotubes, NRG increases the number of surface nAChRs by inducing expression of the α subunit mRNA but has no effect on levels of δ or γ subunits (Harris et al., 1988). In cultured mouse myotubes, NRG most strongly upregulates the nAChR ε subunit, the subunit that is found only in the adult form of the receptor (Martinou et al., 1991). These data suggest that the effects of NRG on different neurotransmitter receptor types are specific to particular subunits whereas other subunits may be regulated by other factors.
Specific effects of different isoforms of NRG on neuronal nACh and GABAA receptor expression have been documented in cultured chick sympathetic neurons (Yang et al., 1998). In these neurons, the form of NRG that contains a cysteine-rich domain in its N terminal (CRD-NRG) increased expression of nAChRs but had no effect on GABA currents. In contrast, the Ig-containing form of NRG, the same form used in our experiments, induced a large increase in GABA-evoked responses but had no effect on ACh currents. Thus, Ig-NRG appears to have similar inductive effects on functional GABAA receptors in sympathetic neurons as in cerebellar granule cells.
NRG and NMDA receptor expression in the cerebellum
In contrast to GABAA receptors, our experiments showed no effects of Ig-NRG on the expression of NR2B and NR2C subunits. However, other investigators, using a different experimental paradigm, reported that Ig-NRG induces the expression of NR2C subunit mRNA. When cerebellar slices dissected at P9 were maintained in control conditions for 7 d and then treated with 5 nm Ig-NRG for 7 d, Ozaki et al. (1997) found a dramatic increase in the levels of NR2C subunit mRNA. In these experiments NR2C expression went from undetectable in the control to high expression in the Ig-NRG-treated slices (Ozaki et al., 1997). As in our experiments, these investigators reported no changes in the NR2B subunit message after exposure to Ig-NRG.
We believe that the differences between our study and the one of Ozaki et al. (1997) most likely reflect fundamental differences in the culture systems. Our experiments were performed on enriched (97% pure) dissociated granule cells, and the observed biological changes in the cells are likely caused by a direct effect of Ig-NRG on the granule neurons. The cerebellar slices, however, contain other cell types in addition to the granule neurons, including other neurons, astrocytes, and oligodendrocytes. Moreover, several of these other cell types, including oligodendrocytes (Vartanian et al., 1994; Canoll et al., 1996) and astrocytes (Pinkas-Kramarski et al., 1994; Anton et al., 1997; Rio et al., 1997), have been shown to express erbB receptors and to respond to NRG. This raises the possibility that the effect of Ig-NRG on NR2C expression in the cerebellar slices may be indirect. Exogenous NRG may cause other cells in the slices, such as astrocytes or oligodendrocytes, to secrete another factor that could then act on the granule cells to upregulate NR2C. Alternatively, it is possible that NRG, by itself, is not sufficient to induce expression of NR2C in granule cells. Rather, another factor, which is present in cerebellar slices but absent from pure granule cell cultures, may act in concert with NRG to induce NR2C expression.
Comparison of the effects of NRG with the effects of BDNF
To study the specificity of NRG we compared the effects of Ig-NRG with the effects of BDNF on granule cells. We found that each factor has a unique set of effects on the granule cells and may therefore regulate different aspects of granule cell development in vivo. In agreement with previous reports, we showed that BDNF acts as a survival factor for granule cells and enhances neurite outgrowth from granule cell reaggregate cultures. We found, however, that BDNF has no effect on the expression of the GABAAreceptor subunits examined. In contrast, Ig-NRG is not a survival factor for the granule cells but like BDNF does increase neurite outgrowth. These results suggest that the erbB and trkB receptors may activate distinct but overlapping intracellular-signaling pathways in granule cell neurons. The most striking and unique effect of Ig-NRG was the upregulation of GABAA receptor β2 subunit expression, which correlated with an increased sensitivity to GABA in the granule cells. Thus, NRG may play a central role in regulating the formation of GABAergic synapses in the CNS and PNS.
An important event in the development of granule cells is the extension of the dendritic processes to form connections with Golgi cells and mossy fibers. Our finding that Ig-NRG greatly enhances neurite outgrowth from granule cells suggests that this factor may regulate dendritic outgrowth from granule cells in vivo. NRG has been shown to enhance neurite outgrowth in other cells as well. NRG can induce neuronal-like differentiation in pheochromocytoma 12 (PC12) cells by increasing both the number of neurites per cell as well as the length of the neurites (Corfas et al., 1994). Similar results were found in PC12 cells overexpressing erbB2 or erbB3 (Gamett et al., 1995). In cultured rat retinal ganglion cells, NRG increased the number of cells with neurites as well as the number of neurites per cell (Bermingham-McDonogh et al., 1996). In other systems, however, endogenous NRG appears to suppress neurite outgrowth. When endogenous NRG is blocked with a soluble form of erbB4 there is an increase in neurite outgrowth either from embryonic day 17 mixed brain cultures or from cells of the P19 neuronal cell line, which have a neuronal phenotype after treatment with retinoic acid (Pinkas-Kramarski et al., 1997). However, the presence of many non-neuronal cell types in these cultures and the fact that the soluble erbB4 may block other erbB4 ligands such as HB-EGF may contribute to the negative effect of NRG on neurite outgrowth in this system.
Many neuronal populations express erbB receptors in the CNS, and many of their afferents express Ig-NRG. Our experiments suggest that Ig-NRG may regulate expression of GABAA receptors in the developing and mature CNS and may therefore play a role in the regulation of inhibitory synaptic transmission.
Footnotes
This work was supported in part by the National Institute of Neurological Disorders and Stroke (NINDS) Grant R01 NS35884 (G.C.), The Klingenstein Foundation (G.C.), The EJLB Foundation (G.C.), National Alliance for Research on Schizophrenia and Depression (G.C.), National Eye Institute Training Grant T32EY07110-05 (H.I.R.), the Mental Retardation Research Center of the National Institutes of Health Grants P30-HD18655 (G.C.) and NS34317 (R.E.S.), and NINDS Grant RO1 NS24830 (H.H.Y.). We thank Amgen for providing Ig-NRG and BDNF, Cari Lai for the erbB4 0615 and 0618 antibodies, Michael Greenberg for the phospho-CREB antibody, and Uri Saragovi for his advice with the MTT assay. We thank M. Bazalakova and M. Kumar for technical assistance and David Zurakowski for help with statistical analysis.
Correspondence should be addressed to Dr. Gabriel Corfas, Division of Neuroscience, Children's Hospital, 300 Longwood Avenue, Boston, MA 02115. E-mail: corfas_g@a1.tch.harvard.edu.
REFERENCES
- 1.Anton ES, Marchionni MA, Lee KF, Rakic P. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997;124:3501–3510. doi: 10.1242/dev.124.18.3501. [DOI] [PubMed] [Google Scholar]
- 2.Beattie CE, Siegel RE. Developmental cues modulate GABAA receptor subunit mRNA expression in cultured cerebellar granule neurons. J Neurosci. 1993;13:1784–1792. doi: 10.1523/JNEUROSCI.13-04-01784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behringer KA, Gault LM, Siegel RE. Differential regulation of GABAA receptor subunit mRNAs in rat cerebellar granule neurons: importance of environmental cues. J Neurochem. 1996;66:1347–1353. doi: 10.1046/j.1471-4159.1996.66041347.x. [DOI] [PubMed] [Google Scholar]
- 4.Benke D, Mertens S, Trzeciak A, Gillessen D, Mohler H. GABAA receptors display association of gamma 2-subunit with alpha 1- and beta 2/3-subunits. J Biol Chem. 1991;266:4478–4483. [PubMed] [Google Scholar]
- 5.Bermingham-McDonogh O, McCabe K, Reh T. Effects of GGF/neuregulins on neuronal survival and neurite outgrowth correlate with erbB2/neu expression in developing rat retina. Development. 1996;122:1427–1438. doi: 10.1242/dev.122.5.1427. [DOI] [PubMed] [Google Scholar]
- 6.Bovolin P, Santi MR, Puia G, Costa E, Grayson D. Expression patterns of gamma-aminobutyric acid type A receptor subunit mRNAs in primary cultures of granule neurons and astrocytes from neonatal rat cerebella. Proc Natl Acad Sci USA. 1992;89:9344–9348. doi: 10.1073/pnas.89.19.9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 1996;17:229–243. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- 8.Connolly C, Wooltorton J, Smart T, Moss S. Subcellular localization of gamma-aminobutyric acid type A receptors is determined by receptor beta subunits. Proc Natl Acad Sci USA. 1996;9:9899–9904. doi: 10.1073/pnas.93.18.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor J, Boileau A, Czajkowski C. A GABAA receptor alpha-1 subunit tagged with green fluorescent protein requires a beta subunit for functional surface expression. J Biol Chem. 1998;273:28906–28911. doi: 10.1074/jbc.273.44.28906. [DOI] [PubMed] [Google Scholar]
- 10.Corfas G, Fischbach GD. The number of Na+ channels in cultured chick muscle is increased by ARIA, an acetylcholine receptor-inducing activity. J Neurosci. 1993;13:2118–2125. doi: 10.1523/JNEUROSCI.13-05-02118.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corfas G, Falls DL, Fischbach GD. ARIA, a protein that stimulates acetylcholine receptor synthesis, also induces tyrosine phosphorylation of a 185-kDa muscle transmembrane protein. Proc Natl Acad Sci USA. 1993;90:1624–1628. doi: 10.1073/pnas.90.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corfas G, Krauss R, Erickson CA, Wietasch K, Plowman G, Fischbach GD. ARIA induces differentiation of PC12 cells. Soc Neurosci Abstr. 1994;20:669. [Google Scholar]
- 13.Corfas G, Rosen KM, Aratake H, Krauss R, Fischbach GD. Differential expression of ARIA isoforms in the rat brain. Neuron. 1995;14:103–115. doi: 10.1016/0896-6273(95)90244-9. [DOI] [PubMed] [Google Scholar]
- 14.Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Kirsky R, Jessen KR. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron. 1995;15:585–596. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 15.Elenius K, Corfas G, Subruto P, Choi CJ, Rio C, Plowman GD, Klagsbrun M. A novel juxtamembrane domain isoform of HER4/erbB4. J Biol Chem. 1997;272:26761–26768. doi: 10.1074/jbc.272.42.26761. [DOI] [PubMed] [Google Scholar]
- 16.Ewert M, de Blas AL, Mohler H, Seeburg PH. A prominent epitope on GABAA receptors is recognized by two different monoclonal antibodies. Brain Res. 1992;569:57–62. doi: 10.1016/0006-8993(92)90368-j. [DOI] [PubMed] [Google Scholar]
- 17.Falls DL, Rosen KM, Corfas G, Lane WS, Fischbach GD. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- 18.Gamett D, Greene T, Wagreich A, Kim H, Koland J, Cerione R. Heregulin-stimulated signaling in rat pheochromocytoma cells. Evidence for ErbB3 interactions with Neu/ErbB2 and p85. J Biol Chem. 1995;270:19022–19027. doi: 10.1074/jbc.270.32.19022. [DOI] [PubMed] [Google Scholar]
- 19.Gault LM, Siegel RE. Expression of the GABAA receptor delta subunit is selectively modulated by depolarization in cultured rat cerebellar granule neurons. J Neurosci. 1997;17:2391–2399. doi: 10.1523/JNEUROSCI.17-07-02391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginty D, Kornhauser J, Thompson M, Bading H, Mayo K, Takahashi J, Greenberg M. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- 21.Harris DA, Falls DL, Dill-Devor RM, Fischbach GD. Acetylcholine receptor-inducing factor from chicken brain increases the level of mRNA encoding the receptor alpha subunit. Proc Natl Acad Sci USA. 1988;85:1983–1987. doi: 10.1073/pnas.85.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmes WE, Sliwkowski MX, Akita RW, Henzel WJ, Lee J, Park JW, Yansura D, Abadi N, Raab H, Lewis GD, Shepard HM, Kuang W-J, Wood WI, Goeddel DV, Vandlen RL. Identification of heregulin, a specific activator of p185erbB2. Science. 1992;256:1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- 23.Khrestchatisky M, MacLennan AJ, Chiang M-Y, Xu W, Jackson MB, Brecha N, Sternini C, Olsen RW, Tobin AJ. A novel alpha subunit in rat brain GABAA receptors. Neuron. 1989;3:745–753. doi: 10.1016/0896-6273(89)90243-2. [DOI] [PubMed] [Google Scholar]
- 24.Laurie D, Seeburg P, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992a;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laurie D, Wisden W, Seeburg P. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992b;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemke G. Neuregulins in development. Mol Cell Neurosci. 1996;7:247–262. doi: 10.1006/mcne.1996.0019. [DOI] [PubMed] [Google Scholar]
- 27.Lindholm D, Dechant G, Heisenberg CP, Thoenen H. Brain-derived neurotrophic factor is a survival factor for cultured rat cerebellar granule neurons and protects them against glutamate-induced neurotoxicity. Eur J Neurosci. 1993;5:1455–1464. doi: 10.1111/j.1460-9568.1993.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 28.Lindholm D, Hamner S, Zirrgiebel U. Neurotrophins and cerebellar development. Perspect Dev Neurobiol. 1997;5:83–94. [PubMed] [Google Scholar]
- 29.Luo X, Kumar M, Siegel R. Expression of GABAA receptor subunits in cultured cerebellar granule neurons is dependent on the β2 subunit. Soc Neurosci Abstr. 1998;24:1832. [Google Scholar]
- 30.Marchionni MA, Goodearl ADJ, Chen MS, Bermingham-McDonogh O, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, Kobayashi K, Wroblewski D, Lynch C, Baldassare M, Hiles I, Davis JB, Hsuam JJ, Totty NF, Otsu M, McBurney RN, Waterfield MD, Strootbent P, Gwynne D. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 31.Martinou J-C, Falls DL, Fischbach GD, Merlie JP. Acetylcholine receptor-inducing activity stimulates expression of the ε-subunit gene of the muscle acetylcholine receptor. Proc Natl Acad Sci USA. 1991;88:7669–7673. doi: 10.1073/pnas.88.17.7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozaki M, Sasner M, Yano R, Lu HS, Buonanno A. Neuregulin-β induces expression of an NMDA-receptor subunit. Nature. 1997;390:691–694. doi: 10.1038/37795. [DOI] [PubMed] [Google Scholar]
- 33.Peles E, Bacus SS, Koski RA, Lu HS, Wen D, Ogden SG, Levy RB, Yarden Y. Isolation of the Neu/HER-2 stimulatory ligand: a 44 kd glycoprotein that induces differentiation of mammary tumor cells. Cell. 1992;69:205–216. doi: 10.1016/0092-8674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- 34.Persohn E, Malherbe P, Richards J. Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J Comp Neurol. 1992;326:193–216. doi: 10.1002/cne.903260204. [DOI] [PubMed] [Google Scholar]
- 35.Pinkas-Kramarski R, Eilam R, Spiegler O, Lavi S, Liu N, Chang D, Wen D, Schwartz M, Yarden Y. Brain neurons and glial cells express Neu differentiation factor/heregulin: a survival factor for astrocytes. Proc Natl Acad Sci USA. 1994;91:9387–9391. doi: 10.1073/pnas.91.20.9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinkas-Kramarski R, Eilam R, Alroy I, Levkowitz G, Lonai P, Yarden Y. Differential expression of NDF/neuregulin receptors ErbB-3 and ErbB-4 and involvement in inhibition of neuronal differentiation. Oncogene. 1997;15:2803–2815. doi: 10.1038/sj.onc.1201466. [DOI] [PubMed] [Google Scholar]
- 37.Plowman GD, Culouscou J, Whitney GS, Green JM, Carlton GW, Foy L, Neubauer MG, Shoyab M. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc Natl Acad Sci USA. 1993;90:1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raetzman LT, Siegel RE. Immature granule neurons from cerebella of different ages exhibit distinct developmental potentials. J Neurobiol. 1999;38:559–570. [PubMed] [Google Scholar]
- 39.Rio C, Rieff HI, Qi P, Khurana TJ, Corfas G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997;19:39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- 40.Sandrock AJ, Dryer S, Rosen K, Gozani S, Kramer R, Theill L, Fischbach G. Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction in vivo. Science. 1997;276:599–603. doi: 10.1126/science.276.5312.599. [DOI] [PubMed] [Google Scholar]
- 41.Sandrock AWJ, Goodearl AD, Yin QW, Chang D, Fischbach GD. ARIA is concentrated in nerve terminals at neuromuscular junctions and at other synapses. J Neurosci. 1995;15:6124–6136. doi: 10.1523/JNEUROSCI.15-09-06124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segal RA, Takahashi H, McKay R. Changes in neurotrophin responsiveness during the development of cerebellar granule neurons. Neuron. 1992;9:1041–1052. doi: 10.1016/0896-6273(92)90064-k. [DOI] [PubMed] [Google Scholar]
- 43.Segal RA, Pomeroy SL, Stiles CD. Axonal growth and fasciculation linked to differential expression of BDNF and NT3 receptors in developing cerebellar granule cells. J Neurosci. 1995;15:4970–4981. doi: 10.1523/JNEUROSCI.15-07-04970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shivers BD, Killisch I, Sprengel R, Sontheimer H, Kohler M, Schofield PR, Seeburg PH. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989;5:703–771. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- 45.Sundstrom P, Deborah S, Sypherd P. Sequence analysis and expression of two genes for elongation factor 1-alpha from the dimorphic yeast Candida albician. J Bacteriol. 1990;172:2036–2042. doi: 10.1128/jb.172.4.2036-2045.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taberno A, Stewart HJS, Jessen KR, Mirsky R. The neuron-glia signal β-neuregulin induces sustained CREB phosphorylation on Ser-133 in cultured rat Schwann cells. Mol Cell Neurosci. 1998;10:309–322. [PubMed] [Google Scholar]
- 47.Vartanian T, Corfas G, Li Y, Fischbach GD, Stefansson K. A role for the acetylcholine receptor-inducing protein ARIA in oligodendrocyte development. Proc Natl Acad Sci USA. 1994;91:11626–11630. doi: 10.1073/pnas.91.24.11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. NeuroReport. 1992;3:1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe M, Mishina M, Inoue Y. Distinct spatiotemporal expressions of five NMDA receptor channel subunit mRNAs in the cerebellum. J Comp Neurol. 1994;343:513–519. doi: 10.1002/cne.903430402. [DOI] [PubMed] [Google Scholar]
- 50.Wisden W. Structure and distribution of multiple GABAA receptor subunits with special reference to the cerebellum. Ann NY Acad Sci. 1995;757:506–515. doi: 10.1111/j.1749-6632.1995.tb17510.x. [DOI] [PubMed] [Google Scholar]
- 51.Yang X, Kuo Y, Devay P, Yu C, Role L. A cysteine-rich isoform of neuregulin controls the levels of expression of neuronal nicotinic receptor channels during synaptogenesis. Neuron. 1998;20:255–270. doi: 10.1016/s0896-6273(00)80454-7. [DOI] [PubMed] [Google Scholar]
- 52.Ymer S, Schofield PR, Draguhn A, Werner P, Kohler M, Seeburg PH. GABAA receptor β subunit heterogeneity: functional expression of cloned cDNAs. EMBO J. 1989;8:1665–1670. doi: 10.1002/j.1460-2075.1989.tb03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshioka A, Ikegaki N, Williams M, Pleasure D. Expression of N-methyl-d-aspartate (NMDA) and non-NMDA glutamate receptor genes in neuroblastoma, medulloblastoma, and other cells lines. J Neurosci Res. 1996;46:164–178. doi: 10.1002/(SICI)1097-4547(19961015)46:2<164::AID-JNR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 54.Zhu XJ, Lai C, Thomas S, Burden SJ. Neuregulin receptors, erbB3 and erbB4, are localized at neuromuscular synapses. EMBO J. 1995;14:5842–5848. doi: 10.1002/j.1460-2075.1995.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]