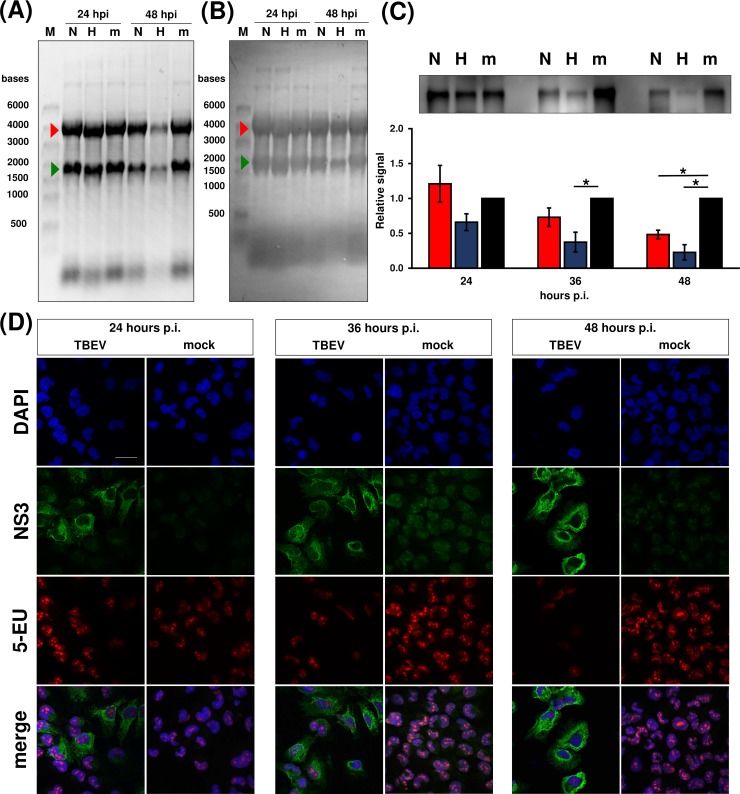
Fig 5. TBEV infection results in decrease of de novo synthesised 45-47S pre-rRNA.
DAOY cells were either infected with Neudoerfl (N) or Hypr strain (H) at MOI 5 or mock-infected (m). Total RNA was isolated at indicated time post infection; 5-ethynyl uridine (1 mM) was added 2 hours before the collection interval. Data are representative of three independent experiments. (A) Integrity of 28S (red arrow) and 18S rRNA (green arrow), evaluated by using in-gel staining with GelRed. (B) Integrity of 28S (red arrow) and 18S rRNA (green arrow), evaluated by methylene blue staining after capillary transfer on PVDF membrane. (C) Upper panel: metabolic labelling of nascent 45-47S pre-rRNA was carried out using Click chemistry and biotin picolyl azide (10 μM) with subsequent chemiluminescent visualisation via biotin-streptavidin-alkaline phosphatase system. Lower panel: values are expressed as mean of three independent experiments with SEM. Significant difference from mock-infected cells was calculated using one-sample Student’s t-test (* P<0.05). (D) In situ metabolic labelling revealed TBEV-induced reduction of nascent RNA at 36 hours p.i. without change in RNA localization. DAOY cells were infected with TBEV Hypr strain (MOI 5) and at indicated time intervals incubated for 2 hours with 1 mM 5-ethynyl uridine (5-EU) in order to label nascent RNA. Detection of incorporated 5-EU was performed by Click reaction using 10 μM biotin picolyl azide followed by fluorescent labelling with streptavidin-DyLight549. Cells were co-stained with anti-NS3 antibodies; signal was further visualised using anti-chicken DyLight488 antibodies. Nuclei were stained with DAPI. Scale bar represents 200 μm.