Abstract
Background and Purpose
Diagnosing small-fiber neuropathy (SFN) is challenging because there is no gold-standard test and few diagnostic tests. This study investigated the clinical symptom profile and its associations with the results of quantitative sensory testing (QST) and the quantitative sudomotor axon reflex test (QSART) as well as the quality of life (QOL) in patients with clinically suspected SFN.
Methods
This study involved 63 patients with clinically suspected length-dependent SFN. Assessments were performed using QST, QSART, SFN Symptoms Inventory Questionnaire, Neuropathic Pain Symptom Inventory, ‘Sirim’ frequency and ‘Sirim’ (cold) pain severity, and 36-item Short-Form Health Survey. Multiple logistic and linear regression analyses were performed to predict risk factors for QST or QSART abnormalities and QOL, respectively.
Results
‘Sirim’ and ‘Sirim’ pain was the most-common (84%) and the most-severe complaint (mean score of 6.3 on a numerical rating scale ranging from 0 to 10) in patients with clinically suspected SFN. The findings of QST [cold detection threshold (CDT)] and QSART were abnormal in 71% (n=45/57) and 62% (n=39/56) of the patients, respectively. An abnormal CDT was correlated with more-severe stabbing pain (odds ratio=2.23, 95% CI=1.02–4.87, p=0.045). Restless-leg symptoms (β=−7.077) and pressure-evoked pain (β=−5.034) were independent predictors of the physical aspects of QOL.
Conclusions
‘Sirim’ pain, similar to cold pain, should be considered a major neuropathic pain in SFN. Among pain characteristics, stabbing pain of a spontaneous paroxysmal nature may be more pronounced in the setting of dysfunctional Aδ fibers with functional autonomic C fibers.
Keywords: small fiber neuropathy, sensory, autonomic, cold, pain, quality of life
INTRODUCTION
Small-fiber neuropathy (SFN) is a peripheral nerve disorder that selectively affects the thinly myelinated Aδ fibers and unmyelinated C fibers, and commonly presents with neuropathic pain, autonomic dysfunction, and hypesthesia to pain and temperature. Symptoms typically show a symmetric length-dependent pattern that develops from the distal part of the body. Many patients report the gradual onset of abnormal sensations in the feet, such as burning pain, electric-shock-like pain, tingling sensation, or pins-and-needles sensation. A non-length-dependent distribution with patchy or proximal involvement has also been reported.1 Autonomic symptoms of varying severities including abnormal sweating, dry eyes, dry mouth, orthostatic dizziness, diarrhea, constipation, and/or erectile dysfunction can occur. Although several causes of SFN have been identified, the etiology is unknown in 30–50% of cases.2 Diabetes mellitus that includes glucose intolerance, autoimmune disease, and sodium channelopathy are known to the most-common underlying causes of pure SFN.2,3
Clinically diagnosing SFN is challenging because the clinical signs of small-fiber damage are often not definite. In addition, routine nerve conduction studies (NCSs) cannot detect abnormalities that are confined to small fibers.4 Diagnosing SFN can be supported by using quantitative sensory testing (QST) to assess the thermal and pain thresholds. However, the visualization and quantification of epidermal nerve fibers in skin biopsies facilitate more-reliable diagnoses of SFN and the recognition of SFN as a distinct clinical entity. The quantitative sudomotor axon reflex test (QSART) could provide additional information about autonomic dysfunction by evaluating the postganglionic sympathetic cholinergic sudomotor function. In Korea, skin biopsies are used to evaluate epidermal nerve fibers in research studies, but QST and QSART are only performed in some tertiary hospitals. Although we commonly encounter patients who are suspected as having SFN, few clinical studies have investigated SFN. In addition, Korean patients frequently report a cold sensation and cold dysesthesia that is called ‘Sirim’ in Korean. However, this symptom is not well characterized and its pathophysiology is currently unknown. There is no equivalent term for ‘Sirim’ in English, but it usually overlaps with an unprovoked unpleasant cold sensation and/or high sensitivity to cold on the skin and/or deeper parts of the body such as the bones and joints. Here we also describe ‘Sirim’ (cold) pain as a ‘Sirim’ sensation that is sufficiently severe to cause pain, and have added it as one of the SFN-associated symptoms.
The objective of the present study was to identify the clinical symptom profile, including their ‘Sirim’ pain, diagnostic test results of QST and QSART, possible etiologies, and quality of life (QOL) in patients with clinically suspected SFN.
METHODS
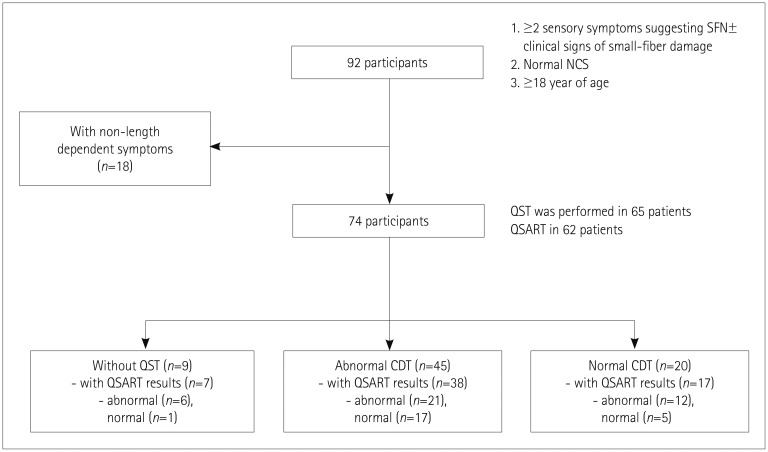
This prospective study was performed between March 2016 and March 2017 at Samsung Medical Center, which is tertiary referral center in Korea. We defined clinically suspected SFN as follows: 1) the presence of at least two sensory symptoms suggestive of SFN, such as pain (e.g., burning, shooting, or prickling), allodynia, lower thermal sensation, hyperesthesia, or paresthesia in which symptoms are length-dependent; 2) abnormal results in either QSART or QST [cold detection threshold (CDT)]; 3) normal NCS findings; and/or 4) signs of small-fiber damage.5 The application of these criteria resulted in the enrollment of 63 consecutive patients with clinically suspected SFN (Fig. 1). The exclusion criteria were 1) the involvement of large nerve fibers or the CNS in neurologic examinations, as indicated by muscle weakness, decreased position/vibration sense, decreased deep tendon reflex, or upper motor neuron signs; 2) the presence of obvious causes, other than SFN, explaining sensory symptoms; 3) an inability to understand and/or sign a written consent form; or 4) being younger than 18 years.
Fig. 1. Flow chart of the selection process to enroll eligible patients. The numbers of finally included patients with abnormal results either for QST (CDT) or QSART are in boldface. CDT: cold detection threshold, NCS: nerve conduction test, QSART: quantitative sudomotor axon reflex test, QST: quantitative sensory testing, SFN: small-fiber neuropathy.
Motor and sensory NCSs were performed using standard surface stimulation and recording techniques6 with in-house normal values to measure the bilateral median, ulnar, peroneal, and tibial motor nerves, and the median, ulnar, and sural sensory nerves. If needed, needle EMG of intrinsic foot muscles (abductor hallucis and extensor digitorum brevis muscles) was performed to rule out radiculopathy.
We assessed SFN by performing QST and QSART. CDT and the warm detection threshold (WDT) were determined by QST on either side of the hand and foot that was more symptomatic using the Computer Aided Sensory Evaluator-IV (WR Medical Electronics, Maplewood, MN, USA). However, we adopted an elevated CDT as the standard for abnormality with an established reference value. Postganglionic sudomotor functions at the forearm, proximal leg, distal leg, and foot on the side with the worst symptoms were assessed by QSART using a Q-Sweat machine (WR Medical Electronics). Some of the patients refused to undergo QST (n=6/63, 9.5%) or QSART (n=7/63, 11%) because of the personal cost to them or for other personal reasons.
SFN-associated symptoms were assessed using the ordinal 13-item Small-Fiber Neuropathy Symptoms Inventory Questionnaire (SFN-SIQ) plus a ‘Sirim’ item scored as follows: 0, never present; 1, sometimes present; 2, often present; and 3, always present. The pain profile was evaluated using the Neuropathic Pain Symptom Inventory (NPSI) questionnaire plus ‘Sirim’ pain. Each of these items was quantified on an 11-point numerical rating scale (NRS) ranging from 0 to 10 for the average pain over the past 24 hours. The bilateral dorsalis pedis and posterior tibial pulses were palpated to exclude peripheral artery disease. In addition, the ankle-brachial index and peripheral vessel ultrasonography were applied to patients with clinical suspicion of vascular insufficiencies. QOL was assessed using the 36-item Short-Form Health Survey (SF-36), with scores ranging from 0 to 100, and higher scores indicating better QOL. Two summary scores of SF-36 were used for the analysis: physical component score (PCS) and mental component score.
To determine the etiology of SFN, we obtained the past medical history and social history, including the use of drugs and alcohol. We performed blood tests including the complete blood count, erythrocyte sedimentation rate, fasting glucose, glycosylated hemoglobin, fasting lipid profile (cholesterol and triglycerides), liver function test, renal function test, thyroid function test (thyroid stimulating hormone, free thyroxine, and triiodothyronine), electrolytes (sodium, potassium, and chlorine), vitamin B12, folate, antinuclear antibodies, antibodies against SSA/SSB and double-strand DNA, rheumatoid factor, angiotensin-converting enzyme, serum protein electrophoresis, and serologies (hepatitis B and C viruses, HIV, and rapid plasma regain). Additional laboratory tests (oral glucose tolerance test, serum immunofixation, α-galactosidase, and/or GLA for Fabry's disease, and abdominal fat biopsy for amyloidosis) were added based on clinical suspicions. Genetic tests for sodium- or potassium-channel mutations were not performed. This study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2015-10-158), and written informed consent was obtained from all participants.
Correlations between scores for SFN-related symptoms (SFN-SIQ items and ‘Sirim’) and neuropathic pain symptoms (NPSI items and ‘Sirim’ pain) were assessed using Spearman's correlation analysis. A correlation was considered to be significant for Spearman's rho (ρ)>0.3 and p<0.01. Logistic regression analysis was performed to evaluate predictors for QST (CDT) or QSART abnormalities after controlling for age, sex, and disease duration. Each NPSI item or each SFN-SIQ item and ‘Sirim’ pain were included as independent variables. Multiple linear regression analysis was used to evaluate risk factors associated with QOL. The presence of possible etiology for SFN was added as an independent variable to the aforementioned independent variables. Group differences (patients with idiopathic vs. nonidiopathic SFN) were analyzed using the chi-square test or Fisher's exact test for categorical variables, and using Student's t test or the Mann-Whitney U test for continuous variables. All data analyses were performed with SPSS (version 20.0, IBM Corp., Armonk, NY, USA) and R software, which is freely available from the CRAN repository (https://cran.r-project.org/).
RESULTS
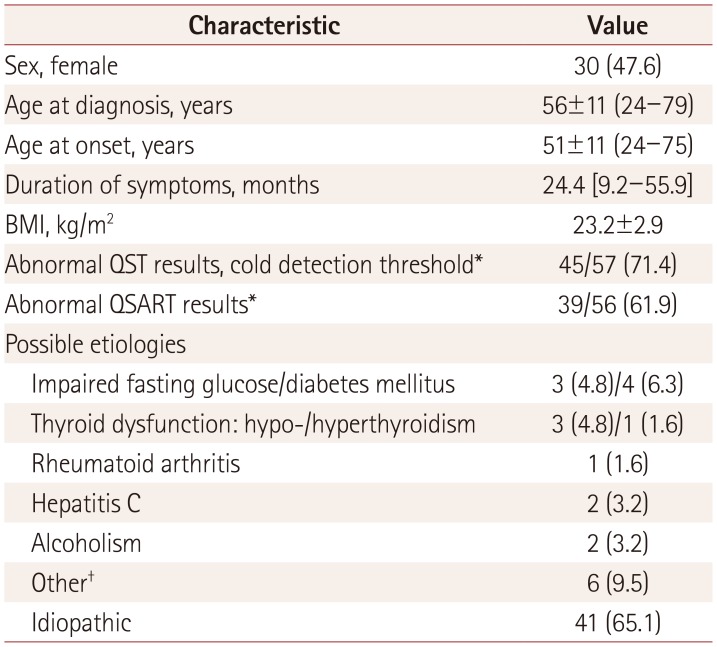
This study included 63 patients (30 females and 33 males) aged 56±11 years (mean±SD) (Table 1). The QST (CDT) and QSART findings were abnormal in 71% (n=45/57) and 62% (n=39/56) of the patients, respectively. The most-common cause was idiopathic, comprising 65% (n=41) of the patients. The most-common known cause was poor glycemic control (11%, n=7), followed by thyroid dysfunction (6%, n=4). In a few cases, autoimmune dysfunction, infections, and alcohol consumption also contributed.
Table 1. Baseline characteristics of the 63 study subjects with clinically suspected small-fiber neuropathy (n=63).
Data are n (%), mean±SD (range), or median [interquartile range] values. *QST and QSART were performed in 57 (90%) and 56 (89%) patients, respectively, †Other includes two cases with positivity for rheumatoid factor, and one case each of positive anti-double stranded DNA IgM, Sjogren's syndrome, monoclonal gammopathy, and a side effect of isoniazid. BMI: body mass index, QSART: quantitative sudomotor axon reflex test, QST: quantitative sensory testing.
SFN symptom profile
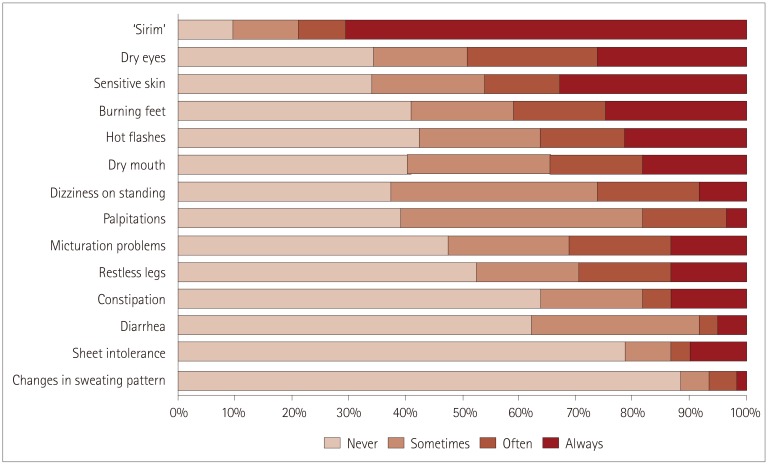
‘Sirim’ was the most-common symptom questionnaire item. Fifty-seven patients (90%) experienced ‘Sirim’ while 70% of them (n=43) always suffered from it (Fig. 2). Other frequently reported symptoms included dry eyes (65%), sensitive skin (65%), and burning feet (59%). Changes in the sweating pattern and sheet intolerance were the least-common symptoms.
Fig. 2. Small-Fiber Neuropathy Symptom Inventory Questionnaire plus ‘Sirim’ findings. The symptoms are listed in order from the most to the least frequent based on the sum of ‘often’ and ‘always’ frequencies.
Neuropathic pain symptom profile
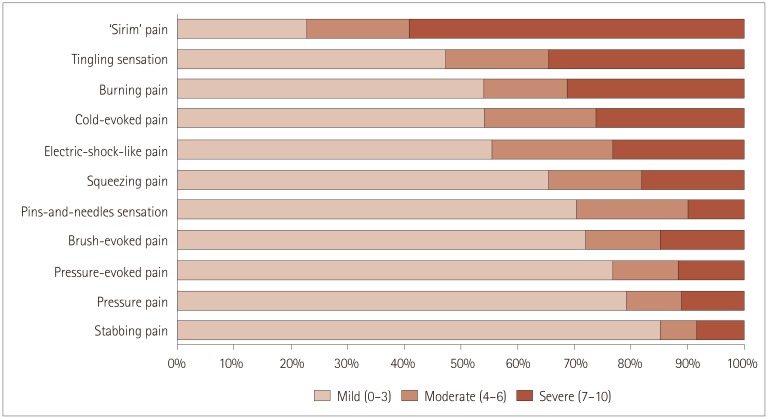
Every patient reported that they experienced pain. Among the pain symptom categories, the most-common and most-severe symptom was ‘Sirim’ pain (NRS score=6.3±3.5, 84% of the patients had ‘Sirim’ pain and 59% of them showed an NRS score of >7) (Fig. 3), followed by tingling sensation (4.3±3.6), cold-evoked pain (3.8±4.1), electric-shock-like pain (3.6±3.3), burning pain (3.3±3.6), squeezing pain (2.9±3.5), and pins-and-needles sensation (2.2±2.9). Among pain symptoms, only the severity of ‘Sirim’ pain was associated with age (ρ=0.316, p=0.013): patients with more-severe ‘Sirim’ pain tended to be older. When we divided patients into two groups using a pain cutoff score of 4 on NRS, patients with higher pain scores (NRS score ≥4, n=47) were older (p=0.012), had longer disease duration (mean=52 months vs. 22 months in patients with NRS scores <4, p=0.008), and possible causes of SFN (47% vs. 0%, p<0.001).
Fig. 3. Neuropathic Pain Symptom InventoryI plus ‘Sirim’ (cold) pain findings. A numerical rating scale ranging from 0 to 10 was used to score pain intensity.
Correlations between SFN-symptom and pain measures
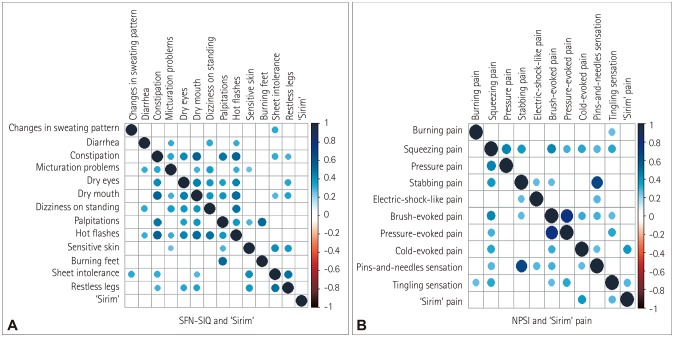
Spearman's correlation analysis revealed weak positive correlations (ρ=0.3–0.5) between SFN-associated symptoms (Fig. 4). The frequencies of autonomic and sensory symptoms tended to be correlated with each other. However, burning feet was only correlated with hot flashes and sheet intolerance, while ‘Sirim’ was not correlated with other symptoms.
Fig. 4. Correlations among SFN-SIQ plus ‘Sirim’ pain (A) and NPSI plus ‘Sirim’ pain (B). Correlations were considered to be significant (and are indicated by blue circles) for Spearman's rho (ρ)>0.3 and p<0.01. NPSI: Neuropathic Pain Symptom Inventory, SFN-SIQ: Small-Fiber Neuropathy Symptoms Inventory Questionnaire.
Most NPSI items exhibited significant correlations with each other. The correlations were strongest between squeezing and pressure pain (ρ=0.492, p<0.001), between stabbing pain and pins-and-needles sensation (ρ=0.559, p<0.0001), and between brush-evoked and pressure-evoked pain (ρ=0.720, p<0.0001). However, ‘Sirim’ pain was only positively correlated with cold-evoked pain (ρ=0.420, p<0.0001) and tingling sensation (ρ=0.248, p<0.0001).
QST and QSART
Fifty patients underwent both QST (CDT) and QSART, whose findings were abnormal in 76% (n=38) and 66% (n=33) of them, respectively. In 38 patients with abnormal CDT, elevated CDT was found in the foot of 26 patients (68%), while 14 patients (out of 26, 54%) also had abnormal CDT in the hand. QSART findings were abnormal in the foot in 24 patients (out of 33 patients with an abnormal result; 73%), while a length-dependent pattern of abnormality (abnormal QSART findings in the foot only; foot and distal leg; and foot, distal leg, and forearm) was found in 15 patients (45%). Global sudomotor dysfunction was found in 1 patient (3%), while regional or patchy sudomotor dysfunction was found in 17 patients (52%).
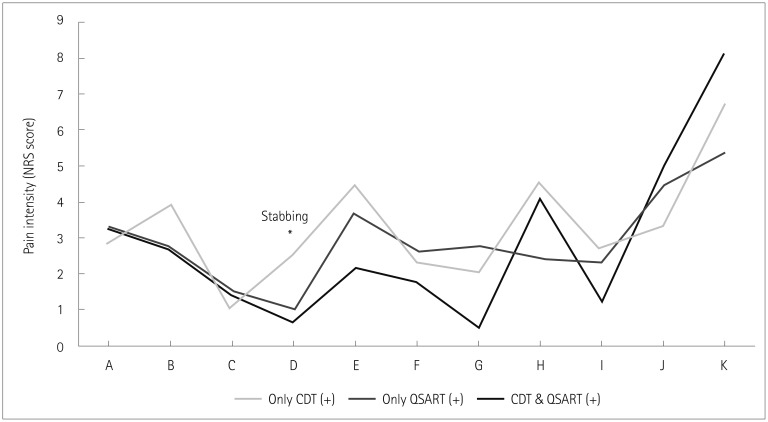
We divided patients into three groups according to CDT and QSART results: 1) abnormal CDT and normal QSART findings (n=17), 2) normal CDT and abnormal QSART findings (n=12), and 3) abnormal CDT and abnormal QSART findings (n=21) (Fig. 5). The stabbing pain was more severe in the group with only abnormal CDT (odds ratio=2.23, 95% CI=1.02–4.87, p=0.045) than in the group with both abnormal CDT and abnormal QSART findings in multinomial multiple logistic regression analysis after controlling for age, sex, and disease duration. There were no significant differences in pain descriptors (NPSI and ‘Sirim’ pain) between the group with only abnormal QSART findings and the group with both abnormal CDT and QSART findings.
Fig. 5. Comparison of pain profiles among patients with different CDT and QSART results. The group with only abnormal CDT findings had more-severe stabbing pain compared to the group with both abnormal CDT and abnormal QSART findings in multiple logistic regression analysis after controlling for age, sex, and disease duration. A: burning pain, B: squeezing pain, C: pressure pain, CDT: cold detection threshold, D: stabbing pain (*), E: electric-shock-like pain, F: brush-evoked pain, G: pressure-evoked pain, H: cold-evoked pain, I: pins-and-needles sensation, J: tingling sensation, K: ‘Sirim’ pain, NRS: numerical rating scale, QSART: quantitative sudomotor axon reflex test.
Idiopathic and nonidiopathic SFN patients
The demographic characteristics, the presence of CDT or QSART abnormality, and QOL (physical and mental aspects) did not differ significantly between patients with idiopathic and nonidiopathic SFN (Supplementary Table 1 in the online-only Data Supplement). However, nonidiopathic SFN patients experienced burning feet and ‘Sirim’ more frequently (uncorrected p=0.011 and p=0.003, respectively), and more-severe brush-evoked pain, tingling sensation, and ‘Sirim’ pain (uncorrected p=0.001, p=0.023, and p=0.004, respectively).
Predictors of QOL
In multiple linear regression analysis, restless-leg symptoms (β=−7.077, p=0.046) and pressure-evoked pain (β=−5.034, p=0.016) were independent predictors of the physical aspects of QOL (R2=0.671). However, there was no significant predictor of the mental aspects of QOL in the patients. Abnormal CDT or QSART findings or the presence of probable etiology was not associated with QOL in patients with clinically suspected SFN.
DISCUSSION
SFN has received increasing attention over the past few years. SFN is a relatively common disorder that often presents with neuropathic pain, especially burning or sharp pain, and dysesthesia that might be difficult to control. In the present study we found that ‘Sirim’ and ‘Sirim’ pain were the most-common and most-severe complaints in patients with clinically suspected SFN. Because ‘Sirim’ refers to a cold sensation in one part of the body that is severe enough for the patient to feel cold throughout, the characteristics of ‘Sirim’ pain are closely related to those of cold pain.
Cold pain has been recognized as one of the SFN-associated symptoms, but its frequency and mechanism remain unclear. A previous study of 67 patients with pure SFN (defined as those with abnormal CDT and/or WDT and reduced density of intraepidermal nerve fibers) found that only 2 patients (3%) described their pain as cold, while 18 patients (26.8.%) reported cold allodynia.7 However, ‘Sirim’ was very common in our study (with 70.5%, 8.2%, 11.5%, and 9.8% of the patients describing it as being always, often, sometimes, and never present, respectively), while cold allodynia (56%) was also frequently accompanied with ‘Sirim.’ Recognition of cold pain as a common symptom of SFN in our study and ethnicity differences compared with the previous study7 might explain such differences. SFN-SIQ does not include cold pain. Among neuropathic pain screening questionnaires, only Neuropathic Pain Questionnaire (NPQ) and Douleur Neuropathique (DN4) contain an item questioning pain of a cold nature.8 Previous studies have suggested that the experience of pain differs among different ethnic groups,9,10 with African Americans showing lower thermal and ischemic pain tolerances than non-Hispanic Whites.10,11 However, few studies have examined other ethnic groups, including Koreans.
Cold pain may result from defective regulation of the skin microcirculation. Intact adrenergic vasoconstrictor nerves and thermally sensitive afferents are required to regulate peripheral blood flow in response to local warming or cooling.4 Hypothermic skin is also frequently found in patients with cold hyperalgesia or allodynia, and either sympathetic denervation supersensitivity or exaggeration of the sympathetic vasoconstrictor outflow mechanism could contribute to this.12 Our study found that cold pain was more severe in older patients, which might be partly due to the presence of cutaneous vasomotor dysfunction.13 In addition, dysfunctional Aδ and nociceptive C fibers can cause an abnormal skin cold sensation and cold pain/allodynia. The characteristics of the cold hypersensitivity differed between patients, which might reflect different underlying neurophysiological mechanisms. Defective cold-specific Aδ afferent input might disinhibit cold-sensitive C-polymodal nociceptive fiber pathways. Thus, paradoxical burning pain could be provoked by innocuous cooling.14 Peripheral sensitization of Aδ fibers or cold-sensitive C fibers also might cause cold hypersensitivity via the abnormal expression or function of sodium, potassium, and calcium channels.15,16 The frequency and severity of ‘Sirim’ and ‘Sirim’ pain were not associated with the CDT or QSART results in our study.
In SFN, Aδ and C fibers might be affected differently during the disease course and possibly also according to the etiology.17 Therefore, diverse individual somatosensory profiles could exist that reflect pathophysiological mechanisms.18 Our study found that an abnormal CDT in the setting of normal QSART findings was associated with more-severe stabbing pain of a spontaneous paroxysmal nature, whereas abnormal QSART findings were not associated with neuropathic pain symptoms. Because Aδ-fiber-mediated pain is usually described as sharp pain of short duration, dysfunctional Aδ fibers—reflected by decreased CDT—might contribute to the severity of stabbing pain. Studies of patients with polyneuropathy have found a positive correlation between deteriorated thermal thresholds and pain intensity.19,20 However, the lack of reports on the correlation between specific neuropathic pain symptoms and single QST parameters means that these findings need to be confirmed.
We adopted QSART as one of diagnostic tools, and 24% of the patients were included due to their QSART findings being abnormal. QSART may have complementary roles in addition to QST (CDT and WDT) and skin biopsy considering the different major neural substrates of the test.2 Small fibers consist of thinly myelinated Aδ fibers that transmit sharp pain and cold impulses, and unmyelinated C fibers that transmit slow pain sensations such as burning pain, warm sensation, and postganglionic autonomic information. Therefore, CDT, WDT, and QSART can evaluate the functioning of Aδ fibers, somatic C fibers, and autonomic C fibers, respectively. A skin biopsy detects the number of intraepidermal nerve fibers, mostly somatic C fibers. Previous studies have found that somatic and autonomic evaluations are independent in the diagnosis of SFN and recommended that concurrent assessments be performed.21,22 Adding QSART to existing SFN criteria that include clinical findings, QST, and skin biopsy was found to increase the diagnostic yield from 38% to 66%.22
Probable underlying causes were not found in most (65%) of the patients in this study even after performing extensive investigations, with the exception of genetic testing for sodium-channel mutations. However, the prevalence rates of idiopathic causes in patients with SFN were also found to be high (≥50%) in other studies.3 Longitudinal assessment and follow-up might be needed to properly identify the cause in individual patients. Patients with a probable etiology tended to experience burning feet and ‘Sirim’ more frequently and more-severe tingling sensation, brush-evoked pain, and ‘Sirim’ pain compared to those with unidentified causes. However, there was no significant difference in their QOL. Restless-leg symptoms and pressure-evoked pain were associated with decreased physical aspects of QOL, explaining about 67% of the physical QOL scores. Another study found that changed sweating pattern, dry eye, and total VAS scores were independent predictors of the PCS in SFN patients, but these predictors only explained 32% of the decrease in QOL.23
This study was subject to several limitations. First, the intraepidermal nerve fiber density—which reportedly has the highest sensitivity and specificity in diagnosing SFN—was not evaluated. Second, clinical signs favoring small-fiber dysfunction, such as pinprick and thermal sensory loss, were not investigated in most patients. Third, genetic tests for sodium-channel mutations were not performed in the etiology workup. Further studies are warranted to determine ethnicity differences in SFN symptoms including cold pain, and to identify the types of impairment of small somatic and autonomic nerve fibers (e.g., nerve density or morphometry) associated with small-fiber damage.
Acknowledgements
This study was supported by academic research funds to EBC provided in 2015 by The Korean Society of Clinical Neurophysiology. This funding source was not involved in designing the study protocol, in the data analysis, or in writing the manuscript.
Footnotes
- Conceptualization: Eun Bin Cho, Byoung Joon Kim.
- Formal analysis: Eun Bin Cho.
- Funding acquisition: Eun Bin Cho.
- Investigation: Eun Bin Cho, Jin Myoung Seok, Ju-Hong Min, Bum Chun Suh, Ki-Jong Park, Byoung Joon Kim.
- Methodology: Eun Bin Cho, Byoung Joon Kim.
- Project administration: Eun Bin Cho, Jin Myoung Seok, Byoung Joon Kim.
- Supervision: Eun Bin Cho, Byoung Joon Kim.
- Validation: Eun Bin Cho, Jin Myoung Seok, Ju-Hong Min, Bum Chun Suh, Ki-Jong Park, Byoung Joon Kim.
- Visualization: Eun Bin Cho.
- Writing—original draft: Eun Bin Cho.
- Writing—review & editing: Eun Bin Cho, Jin Myoung Seok, Ju-Hong Min, Bum Chun Suh, Ki-Jong Park, Byoung Joon Kim.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2019.15.4.480.
Comparison between patients with idiopathic and nonidiopathic SFN
References
- 1.Khoshnoodi MA, Truelove S, Burakgazi A, Hoke A, Mammen AL, Polydefkis M. Longitudinal assessment of small fiber neuropathy: evidence of a non-length-dependent distal axonopathy. JAMA Neurol. 2016;73:684–690. doi: 10.1001/jamaneurol.2016.0057. [DOI] [PubMed] [Google Scholar]
- 2.Terkelsen AJ, Karlsson P, Lauria G, Freeman R, Finnerup NB, Jensen TS. The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes. Lancet Neurol. 2017;16:934–944. doi: 10.1016/S1474-4422(17)30329-0. [DOI] [PubMed] [Google Scholar]
- 3.De Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, Geerts M, Faber CG, Merkies ISJ. Associated conditions in small fiber neuropathy-a large cohort study and review of the literature. Eur J Neurol. 2018;25:348–355. doi: 10.1111/ene.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson JM, Kellogg DL., Jr Local thermal control of the human cutaneous circulation. J Appl Physiol (1985) 2010;109:1229–1238. doi: 10.1152/japplphysiol.00407.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preston DC, Shapiro BE. Electromyography and neuromuscular disorders: clinical-electrophysiologic correlations. 3th ed. Philadelphia: Elsevier Saunders; 2012. [Google Scholar]
- 7.Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008;131:1912–1925. doi: 10.1093/brain/awn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruccu G, Truini A. Tools for assessing neuropathic pain. PLoS Med. 2009;6:e1000045. doi: 10.1371/journal.pmed.1000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell CM, France CR, Robinson ME, Logan HL, Geffken GR, Fillingim RB. Ethnic differences in the nociceptive flexion reflex (NFR) Pain. 2008;134:91–96. doi: 10.1016/j.pain.2007.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med. 2001;63:316–323. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Mechlin MB, Maixner W, Light KC, Fisher JM, Girdler SS. African Americans show alterations in endogenous pain regulatory mechanisms and reduced pain tolerance to experimental pain procedures. Psychosom Med. 2005;67:948–956. doi: 10.1097/01.psy.0000188466.14546.68. [DOI] [PubMed] [Google Scholar]
- 12.Ochoa JL, Yarnitsky D. The triple cold syndrome. Cold hyperalgesia, cold hypoaesthesia and cold skin in peripheral nerve disease. Brain. 1994;117:185–197. doi: 10.1093/brain/117.1.185. [DOI] [PubMed] [Google Scholar]
- 13.Holowatz LA, Thompson-Torgerson C, Kenney WL. Aging and the control of human skin blood flow. Front Biosci (Landmark Ed) 2010;15:718–739. doi: 10.2741/3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campero M, Baumann TK, Bostock H, Ochoa JL. Human cutaneous C fibres activated by cooling, heating and menthol. J Physiol. 2009;587:5633–5652. doi: 10.1113/jphysiol.2009.176040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13:924–935. doi: 10.1016/S1474-4422(14)70102-4. [DOI] [PubMed] [Google Scholar]
- 16.Yin K, Zimmermann K, Vetter I, Lewis RJ. Therapeutic opportunities for targeting cold pain pathways. Biochem Pharmacol. 2015;93:125–140. doi: 10.1016/j.bcp.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 17.Moeller-Bertram T, Schilling JM, Bačkonja MM, Nemenov MI. Sensory small fiber function differentially assessed with diode laser (DL) quantitative sensory testing (QST) in painful neuropathy (PN) Pain Med. 2013;14:417–421. doi: 10.1111/pme.12049. [DOI] [PubMed] [Google Scholar]
- 18.Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain. 2017;158:261–272. doi: 10.1097/j.pain.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krämer HH, Rolke R, Bickel A, Birklein F. Thermal thresholds predict painfulness of diabetic neuropathies. Diabetes Care. 2004;27:2386–2391. doi: 10.2337/diacare.27.10.2386. [DOI] [PubMed] [Google Scholar]
- 20.Ng Wing Tin S, Ciampi de Andrade D, Goujon C, Planté-Bordeneuve V, Créange A, Lefaucheur JP. Sensory correlates of pain in peripheral neuropathies. Clin Neurophysiol. 2014;125:1048–1058. doi: 10.1016/j.clinph.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 21.Thaisetthawatkul P, Fernandes Filho JA, Herrmann DN. Autonomic evaluation is independent of somatic evaluation for small fiber neuropathy. J Neurol Sci. 2014;344:51–54. doi: 10.1016/j.jns.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Thaisetthawatkul P, Fernandes Filho JA, Herrmann DN. Contribution of QSART to the diagnosis of small fiber neuropathy. Muscle Nerve. 2013;48:883–888. doi: 10.1002/mus.23891. [DOI] [PubMed] [Google Scholar]
- 23.Bakkers M, Faber CG, Hoeijmakers JG, Lauria G, Merkies IS. Small fibers, large impact: quality of life in small-fiber neuropathy. Muscle Nerve. 2014;49:329–336. doi: 10.1002/mus.23910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison between patients with idiopathic and nonidiopathic SFN