Abstract
We investigated the association among endometrial hyperemia, uterine bacterial infection, and features of the large ovarian follicles in dairy cows. Genital organs were collected in a complete set at a slaughterhouse, and the degree of endometrial hyperemia was examined for the direct evaluation of uterine inflammation. The rate of bacterial infection in the uterus was higher in cows with endometrial hyperemia regardless of the severity of hyperemia, compared with cows without hyperemia. Moreover, the characteristics of the follicular fluid were changed in cows with uterine bacterial infection and included high concentrations of lipopolysaccharide and malondialdehyde (lipid peroxidation marker). These findings can be utilized as the basic information for the direct evaluation of the uterine inflammatory status in dairy cows.
Keywords: bacterial infection, endometrial hyperemia, lipopolysaccharide, malondialdehyde, ovarian follicle
In postpartum dairy cows, inflammatory diseases of the uterus, such as metritis and endometritis, negatively affect reproductive performance causing significant economic burden [7, 19]. Uterine inflammation occurs due to bacterial infection after calving and is characterized by the accumulation of purulent discharge in the uterus and vagina. For the diagnosis of uterine inflammation, rectal palpation, vaginal examination using the Metricheck device or vaginoscopy, and ultrasonography have been widely applied [7, 12, 18, 20]. However, these methods allow only indirect evaluation of the uterine exudate and provide limited information on the actual inflammation of the endometrium. Thus, direct evaluation of the endometrium is necessary for the accurate diagnosis of uterine inflammation. Endoscopic examination of the uterus (hysteroscopy) has been used as a tool for the diagnosis of uterine inflammation in cows [2, 8]. Madoz et al. (2010) reported that red spots in the endometrium (indicating hyperemia) were identified by hysteroscopy in 33% of the cows that were diagnosed with clinical endometritis on the basis of vaginoscopy. This report suggests that endometrial hyperemia is associated with uterine inflammation. However, little information is available regarding the association of endometrial hyperemia and uterine pathological conditions, such as bacterial infection. Therefore, our study aimed to investigate the relationship between the degree of endometrial hyperemia and pathological status of the uterus by evaluating the presence of bacteria. In addition, the effect of endometrial hyperemia on the follicular function was determined by analyzing the characteristics of the follicular fluid taking under consideration the atretic condition, bacterial toxin accumulation, and oxidative stress status in cows with uterine inflammation.
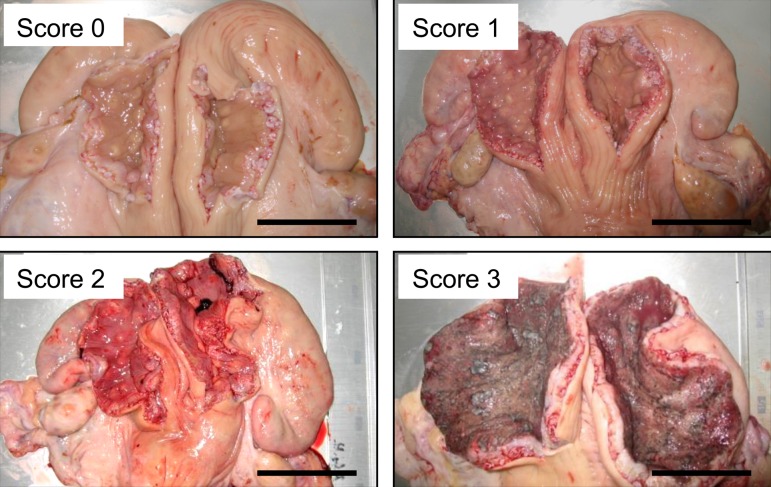
Genital organs of Holstein cows (n=48) were obtained in a complete set at a local slaughterhouse. Immediately after slaughtering, the uterus and ovaries were removed from the animals and transported to the laboratory facility in the slaughterhouse. Animals in the uterine involution process after parturition were excluded from the study by determining the uterine size, shape (left-right symmetry), and condition of placentomes [3]. Also, animals in states of estrus with clear mucus and a preovulatory follicle were not included in the study. For the bacteriological analysis, the surface of the uterus was carefully cleaned and disinfected with 70% ethanol, and the uterine horns were incised for 10 cm along their long axis using sterile surgical scissors, at a distance of approximately 5 cm from the bifurcation of the horns. Subsequently, uterine swabs were obtained from each uterine horn using a sterile swab (BD BBL CultureSwab Plus, Japan Becton Dickinson, Tokyo, Japan) and placed in transport medium within 20 min after slaughtering. During incision, the uterus was grasped with sterile tweezers to avoid contamination by the cow’s blood. Each swab was cultured under both aerobic and anaerobic conditions at a commercial laboratory (Daiichi Kishimoto Clinical Laboratory, Inc., Obihiro, Japan). Endometrial hyperemia was classified into 4 levels by the examination of the appearance of the endometrium, regarding the color, visibility of blood vessels, and presence and degree of swelling. In particular, score 0 referred to no endometrial hyperemia, score 1 referred to endometrium with less than 50% of hyperemic area, score 2 referred to endometrium with more than 50% of hyperemic area, and score 3 referred to endometrium with more than 50% of hyperemic area and erosions. Representative photos of endometria classified according to endometrial hyperemia score are shown in Fig. 1. Endometrial hyperemia scores were not affected by the estrus stage determined by the presence and color of corpus luteum or the follicle size.
Fig. 1.
Representative photos of endometria classified according to the scoring of endometrial hyperemia. Score 0; no hyperemia, score 1; endometrium with 50% of hyperemic area, score 2; endometrium with more than 50% of hyperemic area, score 3; endometrium with more than 50% of endometrial hyperemic area and erosions. Scale bar: 5 cm.
The follicular fluid of large ovarian follicles was aspirated by using a syringe with a 22-gauge needle and centrifuged at 1,500 × g for 1 min at 4°C. The supernatant (follicular fluid) was stored in endotoxin-free glass tubes at −30°C until measurements of lipopolysaccharide (LPS), steroid hormones, or malondialdehyde (MDA) were performed. The concentration of LPS in the follicular fluid was measured as described in our previous study [10]. In particular, the QCL-1000 Chromogenic Limulus Amebocyte Lysate Endpoint Assay Kit (LAL, Lonza Walkersville, Inc., Walkersville, MD, U.S.A.) was used for LPS assessment. The concentrations of estradiol (E2) and progesterone (P4) in the follicular fluid were measured by enzyme immunoassay as previously described [14]. Standard curve ranges were 2−2,000 pg/ml for E2 and 0.05−50 ng/ml for P4. The concentrations of E2 and P4 were obtained from the corresponding standard curve, and the ratio of E2 and P4 concentration (E/P ratio) was calculated and used as an indicator for follicular atresia. The intra- and inter-assay coefficients of variation averaged 5.9 and 3.2% for E2, and 5.6 and 7.8% for P4, respectively. MDA is a marker for lipid peroxidation. Therefore, the concentration of MDA in the follicular fluid was measured as a parameter indicating oxidative stress levels, using the NWLSSTM MDA analysis kit (Northwest Life Science Specialities, Vancouver, Canada) following the manufacturer’s instructions. This assay is based on the reaction of MDA with thiobarbituric acid.
Statistical analyses were performed using Stat View 5.0 (SAS Institute Inc., Cary, NC, U.S.A.). The bacterial detection rate was analyzed by the X2 test. The E/P ratio, and concentrations of LPS and MDA in the follicular fluid were tested for significant differences using ANOVA, followed by the Tukey-Kramer post-hoc test for multiple comparisons. Differences were considered significant at P<0.05. All data are presented as mean ± standard error of the mean.
The bacteria detected in the uterus are shown in Table 1. Bacteria were detected in more cows with endometrial hyperemia scores 1 and 2 compared with cows with score 0 (P<0.05). Cows with score 3 were eliminated from the statistical analysis because of the low number of animals (n=2). Escherichia coli (E. coli) and Streptococcus group G were commonly detected in the uterine fluid of cows that had endometrial hyperemia scores 1, 2 and 3.
Table 1. Bacteria detected in the uterus in cows with endometrial hyperemic scores 0, 1, 2, and 3.
| Endometrial hyperemic score | No. of cows examined | No. of cows with bacteria detection (%) | Detected bacteria (No. of isolates) |
|---|---|---|---|
| Score 0 | 18 | 3 (16.7)a) | Acinetobacter lowffii (2) |
| Enterobacter spp. (1) | |||
| Score 1 | 15 | 8 (53.3)b) | Escherichia coli (3) |
| Enterobacter spp. (1) | |||
| Pasteurella multocida (1) | |||
| Streptococcus group G (1) | |||
| Acinetobacter lowffii (1) | |||
| Klebsiella spp. (1) | |||
| Score 2 | 14 | 7 (50.0)b) | Escherichia coli (3) |
| Enterobacter spp. (1) | |||
| Pasteurella multocida (1) | |||
| Streptococcus group G (2) | |||
| Acinetobacter lowffii (1) | |||
| Stenotrophomonas maltophilia (1) | |||
| Score 3 | 2 | 2 (100) | Escherichia coli (1) |
| Streptococcus group G (1) | |||
a, b) Values with different letters are significantly different within columns (P<0.05).
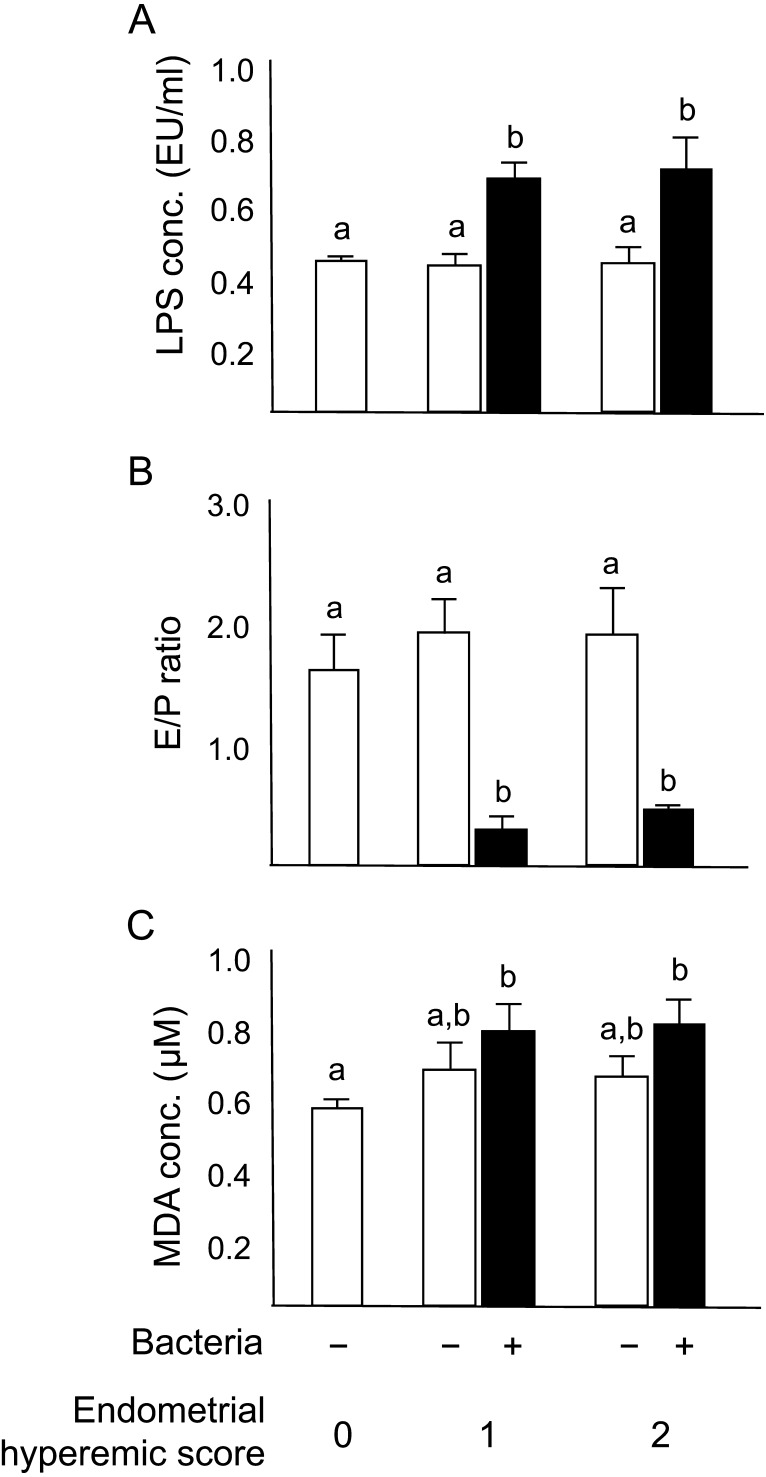
The characteristics of large ovarian follicles are shown in Fig. 2. Cows with endometrial hyperemic score 0 and uterine bacterial infection were eliminated from further analysis because of the low number of animals (n=2). In cows with endometrial hyperemic scores 1 and 2, the concentration of LPS in the follicular fluid of cows with uterine bacterial infection was higher (P<0.05) compared with that in the cows with endometrial hyperemia score 0 that did not have bacteria in their uterus (Fig. 2A). Moreover, in cows with endometrial hyperemic scores 1 and 2, the E/P ratio in the follicular fluid was lower (P<0.05) when bacteria were detected in the uterus compared with that in cows with endometrial hyperemia score 0 that did not have bacteria in their uterus (Fig. 2B). The concentration of MDA in the follicular fluid of cows with uterine bacterial infection and endometrial hyperemia scores 1 and 2 was higher (P<0.05) compared with that in cows with endometrial hyperemia score 0 that did not have uterine bacterial infection (Fig. 2C).
Fig. 2.
Graphs illustrating the measured characteristics of large ovarian follicles (follicular fluid) in cows with different scores of endometrial hyperemia. (A) Lipopolysaccharide concentration, (B) ratio of E2 and P4 concentration (E/P ratio), and (C) malondialdehyde (MDA) concentration in the follicular fluid of cows with (black columns) or without (white columns) uterine bacterial infection. All values are shown as means ± standard error of the means. Values with different letters (a, b) are significantly different between groups (P<0.05).
In the present study, we investigated the association among endometrial hyperemia, uterine bacterial infection, and characteristics of large ovarian follicles in dairy cows. To the best of our knowledge, the present study is the first to provide evidence that the proportion of bacterial detection in the uterus is increased in cows with endometrial hyperemia compared with those that do not have endometrial hyperemia. Moreover, features of the follicular fluid of large follicles such as bacterial endotoxin LPS concentration, atretic condition, and lipid oxidation status were changed in cows with uterine bacterial infection regardless of the degree of uterine hyperemia.
The bacteria detection rate in the uterus was higher in cows with endometrial hyperemic scores 1 and 2 compared with those with score 0 (no hyperemia), although there was no difference between cows with hyperemic scores 1 and 2. This data indicate that the presence of endometrial hyperemia is associated with uterine bacterial infection. However, the severity of the hyperemia is not related with bacterial isolation. It is assumed that cows with endometrial hyperemia, but not bacterial detection might be on the process of recovery from the uterine infection. Most of the bacteria detected in the uterus were potential uterine pathogens or opportunist uterine contaminants, and their pathogenic potential is not commonly associated with uterine lesions [20, 23]. E. coli is one of the most prevalent pathogens causing uterine inflammation and paving the way for other bacteria to colonize the uterus [20, 23]. E. coli was detected in cows with endometrial hyperemic scores 1, 2, and 3 but not in those with score 0, suggesting that E. coli infection in the uterus might be associated with preceding uterine inflammation and possibly result in endometrial hyperemia.
LPS, the cellular component of gram-negative bacteria such as E. coli, has been detected in the follicular fluid in cows with uterine inflammation [5, 9, 10]. Regardless of the endometrial hyperemic score, the follicular LPS concentration increased in cows with uterine bacterial infection, indicating that LPS accumulation in the follicular fluid has relevance to the bacterial infection in the uterus. However, in the present study, LPS was also detected in the follicular fluid of cows that did not have uterine infection by gram-negative bacteria. Our previous study demonstrated that high concentration of LPS was present in the follicular fluid of cows without infection by gram-negative bacteria, suggesting the accumulation of LPS in the follicular fluid long after the inflammation of the uterus had resolved [9]. Therefore, the findings of our previous and present studies indicate that LPS could have been produced by bacteria that had colonized the uterus in the past and then it was accumulated in the follicular fluid and was detectable even after recovery from the infection.
The E/P ratio was used to judge whether the follicles are healthy (E/P ratio >1), or atretic (E/P ratio <1). The decrease in the E/P ratio in cows with uterine bacterial infection suggests that uterine infection might affect the function of ovarian follicles. It must be noted, however, that physiological follicular atresia during the estrus cycles also decrease the E/P ratio. Decreased E/P ratio might be associated with the presence of LPS in the follicular fluid. We have previously shown that follicles with high LPS concentration had decreased E/P ratio and increased mRNA expression of caspase-3, indicating the progress of follicular atresia [10]. In addition, LPS inhibited E2 production by granulosa cells of bovine large follicles in vitro [5, 21].
Diseases in dairy cows, such as mastitis, metritis, and retained fetal membranes, commonly occur during the periparturient period, are known to exhibit oxidative stress [6, 13]. During early lactation in cows, increased production of reactive oxygen species (ROS) coupled with increased concentration of non-esterified fatty acids enhances lipid peroxidation [1]. The measurement of MDA provides a convenient index of lipid peroxidation [16]. Serum MDA concentration was elevated in cows with clinical and subclinical endometritis [4], indicating that uterine inflammation affects the systemic production of MDA. Although there has been no information on the relationship between inflammatory diseases and follicular MDA concentration in cows, the present study demonstrated for the first time that MDA concentration increased in the follicular fluid of cows with uterine bacterial infection. Increased MDA in the follicles of cows with bacterial infection might be due to excessive ROS production by the activated neutrophils in response to the presence of LPS in the follicular fluid. It was previously reported that LPS stimulates ROS production by neutrophils in cows [11]. Moreover, LPS might increase MDA production by reducing the synthesis of E2 in the follicular granulosa cells, since E2 exerts antioxidant activity and inhibits ROS-induced apoptosis of follicular cells [15]. On the other hand, the mediators of oxidative stress might influence the function of granulosa cells and the quality of oocytes [17, 22].
In conclusion, the scoring of endometrial hyperemia was applied for the direct evaluation of the pathology of the uterus, and the presence of endometrial hyperemia was associated with uterine bacterial infection. The follicular characteristics were altered in cows with bacterial infection in the uterus regardless of the severity of hyperemia and included high LPS concentrations of LPS and MDA. These findings can be utilized as the basic information for the direct evaluation of the uterine inflammatory status in dairy cows.
Acknowledgments
This research was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (18H06029) and the Ito Foundation.
REFERENCES
- 1.Bernabucci U., Ronchi B., Lacetera N., Nardone A.2005. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 88: 2017–2026. doi: 10.3168/jds.S0022-0302(05)72878-2 [DOI] [PubMed] [Google Scholar]
- 2.Devine D. A., Lindsay F. E.1984. Hysteroscopy in the cow using a flexible fibrescope. Vet. Rec. 115: 627–628. doi: 10.1136/vr.115.24.627 [DOI] [PubMed] [Google Scholar]
- 3.Gier H. T., Marion G. B.1968. Uterus of the cow after parturition: involutional changes. Am. J. Vet. Res. 29: 83–96. [PubMed] [Google Scholar]
- 4.Heidarpour M., Mohri M., Fallah-Rad A. H., Shahreza F. D., Mohammadi M.2012. Oxidative stress and trace elements before and after treatment in dairy cows with clinical and subclinical endometritis. Rev. Med. Vet. 163: 628–633. [Google Scholar]
- 5.Herath S., Williams E. J., Lilly S. T., Gilbert R. O., Dobson H., Bryant C. E., Sheldon I. M.2007. Ovarian follicular cells have innate immune capabilities that modulate their endocrine function. Reproduction 134: 683–693. doi: 10.1530/REP-07-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kankofer M.2002. Placental release/retention in cows and its relation to peroxidative damage of macromolecules. Reprod. Domest. Anim. 37: 27–30. doi: 10.1046/j.1439-0531.2002.00318.x [DOI] [PubMed] [Google Scholar]
- 7.LeBlanc S. J., Duffield T. F., Leslie K. E., Bateman K. G., Keefe G. P., Walton J. S., Johnson W. H.2002. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J. Dairy Sci. 85: 2223–2236. doi: 10.3168/jds.S0022-0302(02)74302-6 [DOI] [PubMed] [Google Scholar]
- 8.Madoz L. V., De la Sota R. L., Suzuki K., Heuwieser W., Drillich M.2010. Use of hysteroscopy for the diagnosis of postpartum clinical endometritis in dairy cows. Vet. Rec. 167: 142–143. doi: 10.1136/vr.c3157 [DOI] [PubMed] [Google Scholar]
- 9.Magata F., Ishida Y., Miyamoto A., Furuoka H., Inokuma H., Shimizu T.2015. Comparison of bacterial endotoxin lipopolysaccharide concentrations in the blood, ovarian follicular fluid and uterine fluid: a clinical case of bovine metritis. J. Vet. Med. Sci. 77: 81–84. doi: 10.1292/jvms.14-0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magata F., Horiuchi M., Echizenya R., Miura R., Chiba S., Matsui M., Miyamoto A., Kobayashi Y., Shimizu T.2014. Lipopolysaccharide in ovarian follicular fluid influences the steroid production in large follicles of dairy cows. Anim. Reprod. Sci. 144: 6–13. doi: 10.1016/j.anireprosci.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 11.Magata F., Morino I., Teramura M., Tsunoda E., Kawashima C., Haneda S., Miyamoto A., Kida K., Shimizu T.2017. Impact of metritis on the generation of reactive oxygen species by circulating phagocytes and plasma lipopolysaccharide concentration in peripartum dairy cows. Anim. Sci. J. 88: 248–253. doi: 10.1111/asj.12642 [DOI] [PubMed] [Google Scholar]
- 12.McDougall S., Macaulay R., Compton C.2007. Association between endometritis diagnosis using a novel intravaginal device and reproductive performance in dairy cattle. Anim. Reprod. Sci. 99: 9–23. doi: 10.1016/j.anireprosci.2006.03.017 [DOI] [PubMed] [Google Scholar]
- 13.Miller J. K., Brzezinska-Slebodzinska E., Madsen F. C.1993. Oxidative stress, antioxidants, and animal function. J. Dairy Sci. 76: 2812–2823. doi: 10.3168/jds.S0022-0302(93)77620-1 [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto A., Okuda K., Schweigert F. J., Schams D.1992. Effects of basic fibroblast growth factor, transforming growth factor-beta and nerve growth factor on the secretory function of the bovine corpus luteum in vitro. J. Endocrinol. 135: 103–114. doi: 10.1677/joe.0.1350103 [DOI] [PubMed] [Google Scholar]
- 15.Murdoch W. J.1998. Inhibition by oestradiol of oxidative stress-induced apoptosis in pig ovarian tissues. J. Reprod. Fertil. 114: 127–130. doi: 10.1530/jrf.0.1140127 [DOI] [PubMed] [Google Scholar]
- 16.Nielsen F., Mikkelsen B. B., Nielsen J. B., Andersen H. R., Grandjean P.1997. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin. Chem. 43: 1209–1214. [PubMed] [Google Scholar]
- 17.Nuñez-Calonge R., Cortés S., Gutierrez Gonzalez L. M., Kireev R., Vara E., Ortega L., Caballero P., Rancan L., Tresguerres J.2016. Oxidative stress in follicular fluid of young women with low response compared with fertile oocyte donors. Reprod. Biomed. Online 32: 446–456. doi: 10.1016/j.rbmo.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 18.Pleticha S., Drillich M., Heuwieser W.2009. Evaluation of the Metricheck device and the gloved hand for the diagnosis of clinical endometritis in dairy cows. J. Dairy Sci. 92: 5429–5435. doi: 10.3168/jds.2009-2117 [DOI] [PubMed] [Google Scholar]
- 19.Sheldon I. M., Lewis G. S., LeBlanc S., Gilbert R. O.2006. Defining postpartum uterine disease in cattle. Theriogenology 65: 1516–1530. doi: 10.1016/j.theriogenology.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 20.Sheldon I. M., Noakes D. E., Rycroft A. N., Pfeiffer D. U., Dobson H.2002. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction 123: 837–845. doi: 10.1530/rep.0.1230837 [DOI] [PubMed] [Google Scholar]
- 21.Shimizu T., Miyauchi K., Shirasuna K., Bollwein H., Magata F., Murayama C., Miyamoto A.2012. Effects of lipopolysaccharide (LPS) and peptidoglycan (PGN) on estradiol production in bovine granulosa cells from small and large follicles. Toxicol. In Vitro 26: 1134–1142. doi: 10.1016/j.tiv.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 22.Weng Q., Liu Z., Li B., Liu K., Wu W., Liu H.2016. Oxidative stress induces mouse follicular granulosa cells apoptosis via JNK/FoxO1 pathway. PLoS One 11: e0167869. doi: 10.1371/journal.pone.0167869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams E. J., Fischer D. P., Noakes D. E., England G. C., Rycroft A., Dobson H., Sheldon I. M.2007. The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 68: 549–559. doi: 10.1016/j.theriogenology.2007.04.056 [DOI] [PMC free article] [PubMed] [Google Scholar]