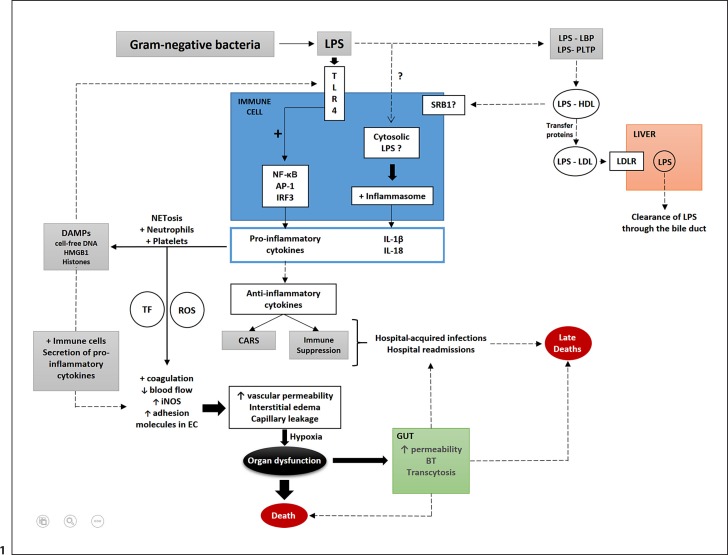
Fig. 1.
Schematic representation of important factors involved in the pathogenicity of septic shock caused by LPS from gram-negative bacteria. LPS from gram-negative bacteria are recognized by TLR4 on immune cells and activate the transcription of genes encoding the proinflammatory cytokines (IL-6, TNF, IFNα, and IL-8) released in the circulation. LPS may reach the cytosol of immune cells (likely mediated by an unknown endocytic pathway). LPS is then recognized by inflammatory caspases that activate the inflammasome, resulting in the release of mature IL-1β and IL-18. Proinflammatory cytokines activate neutrophils, platelets, and NET formation that generate DAMPs (histones, HMGB1, and cell-free DNA). DAMPs are recognized by TLR4 that further stimulates a proinflammatory response. Activated platelets and neutrophils lead to increases in TF and ROS that contribute to changes in endothelial cells (coagulation activation with clot formation, decreases in blood flow, and the upregulation of iNOS, ICAM, and VCAM-1 in endothelial cells. There is also upregulation of adhesion molecules in leukocytes (selectins and integrins). DAMPs also contribute to these effects on endothelial cells by the activation of immune cells, and further release of proinflammatory cytokines. Increased vascular permeability, interstitial edema, and capillary leakage cause tissue hypoxia that can progress to organ dysfunction and death. Intestinal dysfunction occurs mostly by the impairment of intestinal tight junctions and apoptosis, which lead to increases in intestinal permeability, bacterial translocation, and transcytosis. Anti-inflammatory cytokines that are also released into the circulation during sepsis may cause CARS and/or immune suppression. Both, in conjunction with the intestinal alterations in sepsis, contribute to increased rates of secondary and opportunistic hospital-acquired infections and hospital readmissions, factors associated with sepsis-associated late deaths. Alternatively, LPS can be sequestered within lipoprotein fractions in a process mediated by lipid transfer proteins such as LBP and PLTP. Initially, LPS is transferred to HDL and then from HDL to other lipoprotein fractions, such as LDL (again mediated by lipid transfer proteins such as LBP, PLTP, CETP, and possibly BPI). LPS within LDL is taken up by the LDLR and gets cleared from the body through the bile duct. Major pathways (black arrows); secondary pathways (dotted arrows); activation (+); increases (↑); decreases (↓). TLR, toll-like receptor; LPS, lipopolysaccharide; NF-κB, nuclear factor κB; AP-1, activator protein 1; IRF3, interferon regulatory transcription factor 3; IL, interleukin; NET, neutrophil extracellular trap; DAMPs, damage-associated molecular patterns; HMGB1, high-mobility group box 1; TF, tissue factor; ROS, reactive oxygen species; EC, endothelial cell; iNOS, inducible nitric oxide synthase; CARS, compensatory anti-inflammatory response syndrome; BT, bacterial translocation; SRB1, scavenger receptor class B type 1; LBP, lipopolysaccharide binding protein; PLTP, phospholipid binding protein; CETP, cholesteryl-ester transfer protein; BPI, bactericidal/permeability increasing protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LDLR, LDL receptor; ICAM, intercellular adhesion molecule; VCAM-1, vascular cell adhesion molecule.