Bailey et al. review a new neurological channelopathy associated with KCNMA1, encoding the BK voltage- and Ca2+-activated K+ channel.
Abstract
KCNMA1 encodes the pore-forming α subunit of the “Big K+” (BK) large conductance calcium and voltage-activated K+ channel. BK channels are widely distributed across tissues, including both excitable and nonexcitable cells. Expression levels are highest in brain and muscle, where BK channels are critical regulators of neuronal excitability and muscle contractility. A global deletion in mouse (KCNMA1−/−) is viable but exhibits pathophysiology in many organ systems. Yet despite the important roles in animal models, the consequences of dysfunctional BK channels in humans are not well characterized. Here, we summarize 16 rare KCNMA1 mutations identified in 37 patients dating back to 2005, with an array of clinically defined pathological phenotypes collectively referred to as “KCNMA1-linked channelopathy.” These mutations encompass gain-of-function (GOF) and loss-of-function (LOF) alterations in BK channel activity, as well as several variants of unknown significance (VUS). Human KCNMA1 mutations are primarily associated with neurological conditions, including seizures, movement disorders, developmental delay, and intellectual disability. Due to the recent identification of additional patients, the spectrum of symptoms associated with KCNMA1 mutations has expanded but remains primarily defined by brain and muscle dysfunction. Emerging evidence suggests the functional BK channel alterations produced by different KCNMA1 alleles may associate with semi-distinct patient symptoms, such as paroxysmal nonkinesigenic dyskinesia (PNKD) with GOF and ataxia with LOF. However, due to the de novo origins for the majority of KCNMA1 mutations identified to date and the phenotypic variability exhibited by patients, additional evidence is required to establish causality in most cases. The symptomatic picture developing from patients with KCNMA1-linked channelopathy highlights the importance of better understanding the roles BK channels play in regulating cell excitability. Establishing causality between KCNMA1-linked BK channel dysfunction and specific patient symptoms may reveal new treatment approaches with the potential to increase therapeutic efficacy over current standard regimens.
Introduction
Ion channelopathies
Ion channels are ubiquitously expressed throughout the body and perform key membrane transport processes crucial to normal physiological function. Pathogenic alterations to ion channel activity can disrupt homeostatic and physiological functions leading to disorders such as hemiplegic migraines, epilepsy, or cardiac arrhythmias, collectively referred to as "channelopathies” (Brenner and Wilcox, 2012; Kim, 2014; Meredith, 2015). Channelopathies are rare monogenetic disorders stemming from inherited or de novo mutations in genes encoding the functional components of ion channels. Advancements in whole exome sequencing (WES) have contributed to a growing list of diagnosable monogenetic channelopathies (Kim, 2014), yet the molecular basis for these genetic mutations and how they produce clinical phenotypes are not fully established in many cases. Current understandings of channelopathies are limited by several factors, such as deficiencies in (a) the direct evidence for causality due to a very limited number of patients and lack of genetic pedigree analysis, (b) the functional data for the classification of mutant channel properties, and (c) the tissue loci and nature of aberrant excitability linked to abnormal patient phenotypes. This review will focus on one channelopathy involving the large conductance Ca2+ and voltage-activated K+ (BK, KCa1.1) channel, encoded by KCNMA1. The hallmark clinical presentation of KCNMA1-linked channelopathy is neurological dysfunction, including seizures, movement disorders, developmental delay, and intellectual disability.
BK channel structure
The KCNMA1 gene, located on human chromosome 10q22.3, produces the pore-forming α-subunit of the BK channel (Dworetzky et al., 1994; Pallanck and Ganetzky, 1994; McCobb et al., 1995). BK channels, named for their “Big K+” conductance (>100 pS), are members of the voltage-gated K+ channel family and mediate K+ efflux from excitable and non-excitable cells (Latorre and Miller, 1983; Neyton and Miller, 1988). BK channels assemble as homotetramers of the KCNMA1 gene product. Each α-subunit consists of seven transmembrane domains (S0–S6), and a large intracellular C-terminus (Fig. 1). The extracellular N-terminus and presence of the S0 transmembrane segment differs between BK and other voltage-gated K+ channels and confers interactions with accessory proteins (β and γ subunits; Wallner et al., 1996; Morrow et al., 2006; Yan and Aldrich, 2010, 2012). The S1–S4 segments contain positively charged residues and constitute the voltage sensing domain, while the S5–S6 segments form the pore domain, which houses the highly K+-selective conduction pathway (Meera et al., 1997; Stefani et al., 1997; Horrigan et al., 1999; Horrigan and Aldrich, 2002; Yang et al., 2015). The intracellular C-terminus of the BK channel encompasses the gating ring, a structure comprised of two regulators of K+ conductance (RCK) domains. RCK1 and RCK2 each contain two distinct high-affinity Ca2+ binding sites, mediating the allosteric gating of BK channels (Xia et al., 2002; Wang and Sigworth, 2009; Lee and Cui, 2010; Yuan et al., 2010, 2011; Zhang et al., 2010). The human BK channel structure was determined in 2010 (Yuan et al., 2010), providing a basis for understanding the location of specific residues, such as mutations, with respect to functional domains.
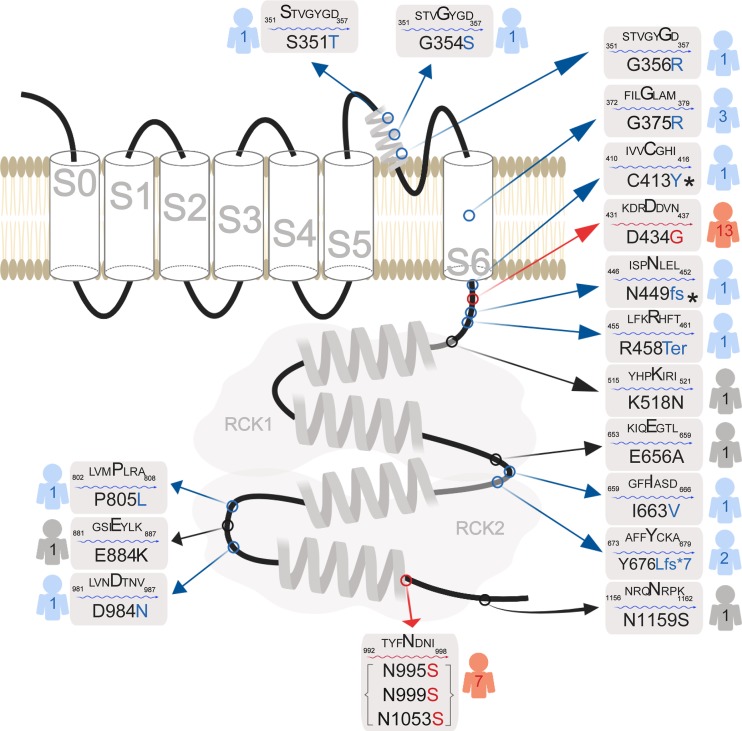
Figure 1.
Human KCNMA1 mutations. Schematic of the KCNMA1 gene product, the α-subunit of the BK channel (GenBank accession no. NM_002247.3). The voltage-sensitive pore-forming region of the BK channel is comprised of transmembrane domains S0–S6, while the intracellular gating ring contains the two Ca2+ binding sites in the RCK1 and RCK2 domains. Red indicates GOF mutations (n = 2), blue indicates LOF or putative LOF mutations (n = 11), and black indicates putative benign mutations (n = 3) or VUS (n = 1). *C413Y/N499fs is a double mutation harbored by a single patient. Numbers to the right or left of each mutation indicate the total number of patients carrying each mutation reported in published studies. N995S, N999S, and N1053S are the same amino acid substitution, but are reported in the literature using three different reference sequencing number schemes.
Voltage and calcium regulation of BK channel activity
Multiple activation mechanisms are involved in opening the channels in vivo (Hou et al., 2009). Under physiological conditions in excitable cells, the BK channel activation requires both membrane depolarization and an increase in intracellular Ca2+ to micromolar levels for activation (Yang et al., 2015). These high concentrations of Ca2+ are attained in local microdomains through tight functional coupling with voltage-gated Ca2+ channels or channels that release Ca2+ from intracellular stores (Fakler and Adelman, 2008). Different tissues exhibit BK currents of varying voltage and Ca2+-dependent activation properties, underlying the complex roles for BK channels in regulating excitability. This cell and tissue-specific regulation is achieved via extensive alternative splicing of KCNMA1 transcripts, assembly with modulatory β- and γ-regulatory subunits, and a variety of post-translational modifications (Kyle and Braun, 2014; Latorre et al., 2017; Gonzalez-Perez and Lingle, 2019). These mechanisms work together to tailor the voltage and Ca2+ dependence of channel activation, as well as the kinetics of activation and deactivation gating, for the cell-specific function of BK channels in excitability or K+ transport.
Tissue distribution and links to human pathophysiology
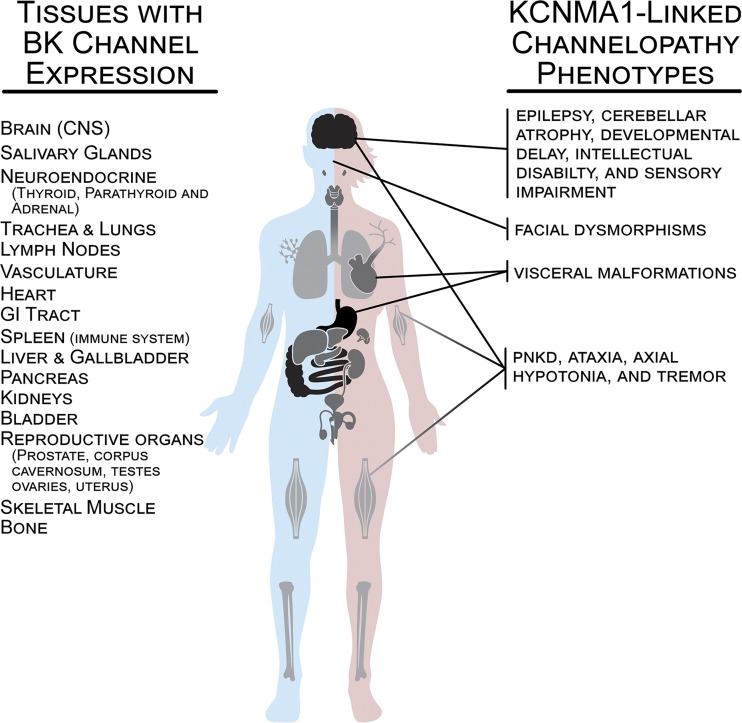
In humans, BK channels are widely expressed in the body including nervous, muscular, skeletal, endocrine, cardiovascular, digestive, urinary, and reproductive systems (Fig. 2; Pallotta et al., 1981; Adams et al., 1982; Becker et al., 1995; McCobb et al., 1995; Hirukawa et al., 2008; Cui et al., 2009; Fagerberg et al., 2014; Contet et al., 2016; Latorre et al., 2017; Gonzalez-Perez and Lingle, 2019). Prior to the identification of KCNMA1-linked channelopathy patients, changes in BK channel expression or activity had been implicated in human studies of erectile dysfunction, overactive bladder, hypertension, and premature uterine contraction during pregnancy (Christ et al., 2001, 2009; Melman et al., 2007; Tomás et al., 2008; Grimm and Sansom, 2010; Yang et al., 2013; Li et al., 2014). Moreover, several nonsynonymous single nucleotide polymorphisms (SNPs) in KCNMA1 and genes encoding BK-specific auxiliary β subunits (KCNMB1-4) were also linked to human disorders or disease risk, including autism, hypertension and cardiovascular function, and asthma (Gollasch et al., 2002; Schuckit et al., 2005; Laumonnier et al., 2006; Han et al., 2013). Despite these studies implicating BK channel function in several pathological conditions, genetic linkage and analysis of human tissues have revealed only a limited picture of what is known about BK channel roles in vivo. Extensive investigations in animal models, which are more accessible to the interrogation of specific cell types and tissues, have provided a more complete assessment of the various roles for BK channels in physiology and pathophysiology. Although neurological and muscle phenotypes are well represented, BK channel function in rodents is more broadly required for a panoply of organ functions that parallels their wide expression across tissues (Fig. 3). Taken together, these animal studies detail a greater potential for wide-ranging pathophysiological dysfunction in patients carrying mutations in KCNMA1.
Figure 2.
BK channel expression in human tissues and prominent phenotypes reported in patients with KCNMA1-linked channelopathy. Major tissues or systems expressing BK channels are depicted in black (high relative expression), gray (medium), and light gray (low). Organs demonstrating high levels of BK channel expression include the CNS (olfactory system, neocortex, basal ganglia, hippocampus, thalamus, habenula and its tract to the interpeduncular nucleus in the midbrain, cerebellum, vestibular nuclei in the hindbrain, and spinal cord), gastrointestinal tract (stomach, small intestine, and colon), and reproductive organs (corpus cavernosum, prostate, testes, ovaries, and uterus). Organs demonstrating medium BK channel expression include salivary glands, neuroendocrine glands (thyroid, parathyroid, adrenal), heart, urinary bladder, liver and gallbladder, kidneys, and spleen/immune system. Organs demonstrating low levels of BK channel include lungs, lymph nodes, vasculature, skeletal muscle, and bone. Data on expression levels were derived from the Human Protein Atlas v18.1 (https://www.proteinatlas.org; Uhlén et al., 2015), the NCBI Gene Database (https://www.ncbi.nlm.nih.gov), and published reports (Dworetzky et al., 1994; McCobb et al., 1995; Brenner et al., 2000).
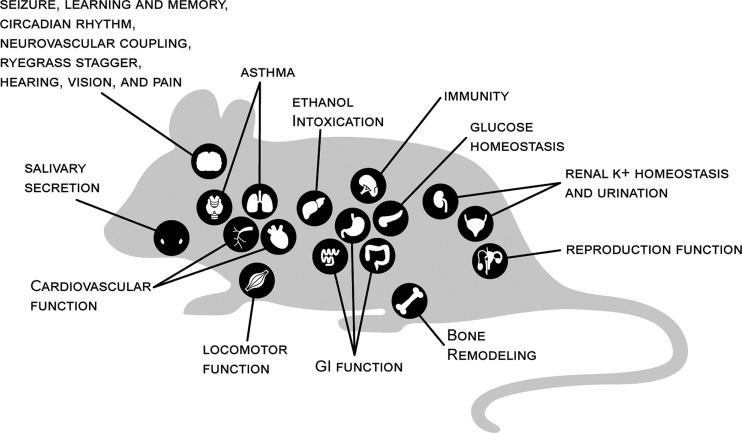
Figure 3.
BK channel dysfunction by organ and functional system in rodent models. BK channel knockout mice (KCNMA1−/−) show a wider range of pathophysiology compared with human KCNMA1 patients with LOF mutations. Such findings are consistent with the extensive distribution of BK channels across tissues. Key organ and functional systems disrupted by BK channel dysfunction in mouse models are indicated. BK channel activity was required for several aspects of neurological operations in rodent models, such as regulation of neuronal excitability (Jin et al., 2000; Faber and Sah, 2003; Brenner et al., 2005; Shruti et al., 2008; Sheehan et al., 2009), locomotor function (Meredith et al., 2004; Sausbier et al., 2004; Chen et al., 2010), circadian rhythm (Meredith et al., 2006; Kent and Meredith, 2008; Montgomery et al., 2013; White et al., 2015; Whitt et al., 2016), learning and memory (Typlt et al., 2013b), vision (Henne and Jeserich, 2004; Grimes et al., 2009; Tanimoto et al., 2012), hearing and vestibular reflexes (Pyott et al., 2007; Maison et al., 2013; Rohmann et al., 2015; Pyott and Duncan, 2016; Nelson et al., 2017), and neurovascular coupling (Filosa et al., 2006; Girouard et al., 2010). In addition to neurological roles, rodent models further revealed that BK channels are required for regulation of cardiovascular function (Sausbier et al., 2005; Imlach et al., 2010; Lai et al., 2014; Nagaraj et al., 2016), airway control (Sausbier et al., 2007; Goldklang et al., 2013; Manzanares et al., 2014), urination (Meredith et al., 2004; Thorneloe et al., 2005; Brown et al., 2008; Sprossmann et al., 2009), glucose homeostasis (Houamed et al., 2010; Düfer et al., 2011), renal K+ homeostasis (Liu et al., 2007; Rieg et al., 2007), reproductive function (Werner et al., 2005, 2008; Li et al., 2014), ethanol intoxication (Martin et al., 2004; Pietrzykowski et al., 2008), gastrointestinal function (Sausbier et al., 2006a; Sørensen et al., 2008), body weight (Halm et al., 2017), pain (Hayashi et al., 2016), immunity (Essin et al., 2009), bone remodeling (Sausbier et al., 2011; Hei et al., 2016), and salivary secretion (Maruyama et al., 1983; Stummann et al., 2003).
KCNMA1 patient mutations
16 KCNMA1 mutations in 37 symptomatic patients have been reported in the literature to date (Fig. 1). Functional classifications into GOF and LOF effects on channel properties have been based on expression of BK channel complementary DNAs harboring the patient mutations in nonexcitable heterologous cell lines (Table 1 and Supplemental text). From these studies, two mutations have been shown to confer GOF properties to BK channels: D434G and N995S (also referred to as N999S or N1053S; Du et al., 2005; Zhang et al., 2015; Li et al., 2018; Plante et al., 2019). 10 mutations have been classified as LOF (S351Y, G354S, G356R, G375R, C413Y/N449fs, I663V, P805L, and D984N; Carvalho-de-Souza et al., 2016; Liang et al., 2019) or putative LOF (premature truncation mutations: Y676Lfs*7 and Arg458Ter; Tabarki et al., 2016; Yeşil et al., 2018). The remaining mutations have not yet been conclusively defined with respect to channel properties (variant of unknown significance, VUS; E884K; Zhang et al., 2015) or have not been demonstrated to produce changes in channel function (K518N, E656A, and N1159S; Li et al., 2018).
Table 1. KCNMA1 mutation effects on BK channel function.
| Mutation | BK current properties | Mechanism |
|---|---|---|
| D434G | Increased current | G-V shift to hyperpolarized potentials, increased open probability, faster activation, slower deactivation, increased Ca2+ sensitivity (Du et al., 2005; Díez-Sampedro et al., 2006; Wang et al., 2009; Yang et al., 2010; Plante et al., 2019) |
| N995/999/1053S | Increased current | G-V shift to hyperpolarized potentials, increased open probability, faster activation, slower deactivation, Ca2+-independent mechanism (Li et al., 2018; Plante et al., 2019) |
| S351Y, G356R, G375R, N449fs*, I663V | No current | Not determined (Liang et al., 2019) |
| C413Y, P805L | Reduced current | G-V shift to depolarized potentials, decreased expression (P805L; Liang et al., 2019) |
| D984N | Reduced current | Not determined (Liang et al., 2019) |
| G354S | Reduced current | Slower activation (Carvalho-de-Souza et al., 2016) |
| R458Ter, Y676Lfs*7 | Not determined | Putative truncations (Tabarki et al., 2016; Yeşil et al., 2018) |
| K518N, E656A, N1195S | No current difference | Li et al., 2018 |
| E884K | Not determined | Zhang et al., 2015 |
Dark gray, GOF mutations; light gray, LOF mutations; no shading, VUS. Full descriptions of experimental investigations for mutant channel properties are contained in Supplemental text. The N995S/N999S/N1053S mutation is reported in the literature using three different reference sequence numbering schemes but constitutes the same residue substitution (Figure 1). In this review, this mutation will be referred to by the numbering scheme in the original publication for the data being discussed.
The majority of the mutations are de novo variants, and patients are heterozygous (Fig. 1). Since BK channels are comprised of tetramers of the KCNMA1 gene product, this presents the potential for mitigation of mutant effects by coassembly with WT subunits in heterozygous patients. However, it is not yet known whether tetramers are actually formed between WT and mutant subunits in patients with one normal and one mutant allele, or how the interaction between these subunits would affect BK channel properties. It is also not well studied how the native regulatory processes that set channel function in vivo would combine with the mutation to affect channel properties. It is also possible that some previously established GOF or LOF classifications could change under different experimental conditions than those initially tested. KCNMA1 patient mutations that have been characterized in at least one standard condition are presented in Table 1 and further described in the Supplemental text.
Neuronal mechanisms of BK channel dysfunction
The mechanisms by which KCNMA1 mutations alter BK channel activity to cause patient symptoms is still an open question. In the central nervous system (CNS), BK channels play an important role in neuronal excitability, passing outward K+ current upon membrane depolarization and increased intracellular Ca2+ during the action potential, leading to hyperpolarization of the membrane and decreased excitability (Hille, 2001). BK current shapes action potential waveforms by mediating the repolarization and afterhyperpolarization (AHP) phases (Shao et al., 1999; Faber and Sah, 2002; Gu et al., 2007; Ly et al., 2011; Contet et al., 2016). In the majority of neuronal and muscle contexts where the channels are expressed, activation of BK current reduces neuronal firing rates, presynaptic neurotransmitter release, or muscle contraction or tone (Jaggar et al., 2000; Contet et al., 2016; Tricarico and Mele, 2017). In neurons, this decrease in firing is predominantly due to the contribution of BK current to the AHP phase of the action potential (Storm, 1987; Contet et al., 2016). In cerebellar Purkinje and dentate gyrus (DG) granule neurons, cell types which have been linked to central neurological phenotypes, the β4 subunit facilitates the suppressive role for BK channels in neuronal firing. β4 subunits slow BK current activation, shifting their activation to the AHP phase (Brenner et al., 2005; Womack et al., 2009; Petrik et al., 2011; Benton et al., 2013). BK channel activation also reduces excitatory synaptic activity at neuromuscular junctions and in central neurons (Robitaille et al., 1993; Raffaelli et al., 2004). In smooth muscle cells, BK channels hyperpolarize the membrane potential, which in turn decreases Ca2+ influx through voltage-gated Ca2+ channels and promotes smooth muscle relaxation (Jaggar et al., 2000). These studies suggest that LOF in BK channel activity would broadly produce hyperexcitability, such as that resulting in seizure. Consistent with this, at least half of the patients with putative LOF KCNMA1 mutations have seizures of some type (Fig. 4).
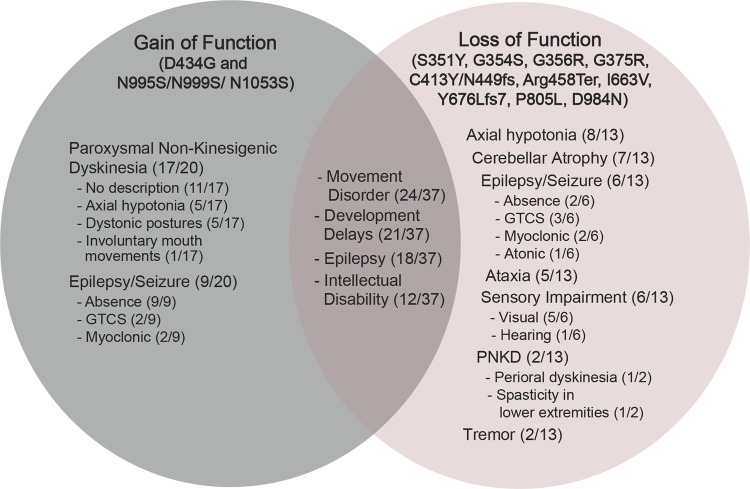
Figure 4.
Patient phenotypes for GOF versus LOF KCNMA1 alleles. Summary of the main phenotypes exhibited by KCNMA1-linked channelopathy patients: seizure, movement disorder, neurodevelopment, and intellectual disability. The denominator is the total number of patients included in each mutation group, and the numerator is the number of patients reported with the particular phenotype. The intersection includes shared symptoms among all patients (n = 37) identified with any KCNMA1 mutation (n = 16) reported in this review. Additional subtype descriptors for PNKD, epilepsy, and sensory impairment are annotated. 11/20 of GOF patients had no additional description for their PNKD symptoms in the published reports.
GOF KCNMA1 patient mutations are also clearly associated with seizure, raising the possibility that changes in BK channel activity in both directions could alter the balance of excitation and inhibition in the brain. Underlying the potential for bidirectional effects on excitability, BK channels are expressed in both excitatory and inhibitory neurons, and the effects of BK current on firing can vary by neuron type (Contet et al., 2016). Activation of BK current can both decrease and increase neuronal firing rates (Montgomery and Meredith, 2012), a paradox that can be resolved by understanding the voltage and Ca2+ dependence and gating kinetics of the channels in a specific membrane and cellular context. Predicting the effect of BK channel activation on action potential frequency, for example, requires knowledge of the activation, inactivation, and deactivation properties for the BK channels, as well as their subcellular localization and interaction with other ionic currents in that cell (Jaffe et al., 2011; Ly et al., 2011; Montgomery and Meredith, 2012; Bock and Stuart, 2016; Contet et al., 2016). The intrinsic gating properties, in turn, are regulated by the subunit composition of the BK channel complex: the α, β, and γ subunits of the BK channel and its associated Ca2+ source (Berkefeld and Fakler, 2008; Latorre et al., 2017). A pro-excitatory effect of BK channel activation is revealed by BK antagonists that decrease neuronal firing or plateau potentials (Solaro et al., 1995; Jin et al., 2000; Van Goor et al., 2001; Sausbier et al., 2004; Gu et al., 2007; Bell et al., 2008) or reduce sinoatrial node firing and heart rate (Lai et al., 2014), an opposite effect from the typical result of inhibiting BK channels in most excitable cells. Furthermore, BK channel inhibition has been reported to exert anticonvulsant, rather than epileptogenic, effects in picrotoxin and pentylenetetrazole (PTZ) mouse seizure models (Sheehan et al., 2009).
The mechanisms underlying the pro-excitatory effects could simply stem from the increased BK current produced by GOF channels hyperpolarizing the membrane of inhibitory neurons. On the other hand, one property correlated with pro-excitatory BK currents is fast activation. More rapid BK current activation directly increases neuronal firing rates by leading to faster repolarization of the action potential (Jaffe et al., 2011; Ly et al., 2011; Montgomery and Meredith, 2012; Contet et al., 2016), an effect that could link GOF channels to hyperexcitation and increased firing in excitatory neurons. In addition, rapidly activating BK currents contribute to the fast phase of the AHP, facilitating enhanced Nav channel recovery from inactivation and decreased activation of other K+ currents (Van Goor et al., 2001; Gu et al., 2007; Gittis et al., 2010; Wang et al., 2014). Thus, rather than strictly hyperpolarizing membranes due to increased BK current, mutations such as D434G and N995S/N999S/N1053S, which activate more rapidly than WT channels (Du et al., 2005; Wang et al., 2009; Yang et al., 2010; Li et al., 2018; Plante et al., 2019), may be capable of directly increasing action potential firing. Thus, the bidirectional and cell-specific effects of BK channels on neuronal excitability suggests that KCNMA1 mutation-linked dysfunction could disrupt neuronal circuitry and provoke pathological symptoms via multiple potential mechanisms.
KCNMA1-linked channelopathy and the spectrum of patient phenotypes
The clinical symptoms of patients with KCNMA1-linked channelopathy have not been comprehensively defined but are principally characterized by seizures and movement disorders. Of the 16 KCNMA1 mutations reported in the literature to date, 4 mutations have been identified in more than one patient (Fig. 1). Comparison of these cases suggests that patients sharing the same mutation do exhibit similar and/or overlapping neurological and neuromuscular phenotypes, in addition to distinct symptoms. Particularly with the de novo mutations, this provides evidence for the causative nature of the mutations in patient symptoms. The evidence for symptomatic similarities by mutation is summarized in the following.
The D434G KCNMA1 mutation was identified in 13 members of a large family with symptoms of a coexistent syndrome of generalized epilepsy and paroxysmal dyskinesia (Du et al., 2005). Within this group, seven patients developed PNKD, one patient developed epilepsy, and five patients developed both symptoms (Du et al., 2005). These clinical findings were the first to link KCNMA1 to disease in humans and provided prima facie confirmation of the BK channel’s extensive involvement in CNS function.
The N1053S mutation (also called N995S or N999S) has been identified in seven unrelated patients from around the world (Zhang et al., 2015; Wang et al., 2017; Li et al., 2018; Heim et al., 2019) who share similar symptoms with the D434G patients. Of these patients, four developed early onset PNKD, two developed epileptic seizures, and one patient developed both symptoms. In addition, all seven patients were reported to have developmental delays, three patients had intellectual disability, and one patient had other symptoms involving CNS dysfunction, potentially expanding the neurological phenotypes associated with this channelopathy. The symptoms associated with D434G and N1053S mutations constitute the description of phenotype MIM #609446 (“PKND3”) provided by the Online Mendelian Inheritance in Man (OMIM) database (https://omim.org).
The Y676Lfs*7 mutation was identified in two siblings from a consanguineous family (Tabarki et al., 2016). Both patients were reported to have the same symptoms starting at a young age which included epileptic seizures, severe developmental delay, and nonprogressive cerebellar atrophy (Tabarki et al., 2016). These two patient cases constitute the description of phenotype MIM #617643 provided by the OMIM database. While seizure and developmental delay are shared phenotypes with D434G and N995S/N999S/N1053S, cerebellar atrophy without paroxysmal dyskinesia is a distinct phenotype and adds to the variability of neurological symptoms associated with this channelopathy.
The final mutation reported in the literature that is shared by multiple patients is G375R (Liang et al., 2019). This mutation was identified in three unrelated patients who experienced overlapping and heterogenous symptoms. All three patients were reported to have a multiple malformation syndrome characterized by facial dysmorphisms, visceral malformations, development delay, intellectual disability, and axial hypotonia (Liang et al., 2019). Additionally, two of the three patients developed epileptic seizures and one patient had mild cerebral and cerebellar atrophy (Liang et al., 2019). This mutation is associated with the most distinct clinical findings relative to the other three mutations but still demonstrates some phenotypic overlap with the previous mutations regarding neurological and neuromuscular dysfunction.
Of these four KCNMA1 mutations, D434G exhibited autosomal dominant inheritance and was the only mutation identified in patients from successive generations of a pedigree (Du et al., 2005). Y676Lfs*7 demonstrated autosomal recessive inheritance and was identified in a single generation of a pedigree (Tabarki et al., 2016). These hereditable mutations produced very similar symptoms in affected family members, suggesting a causal role. The remaining two mutations shared by multiple patients were de novo variants (N995S/N999S/N1053S and G375R). In these cases, clinical similarities between patients sharing the same mutations has suggested causality in the absence of comprehensive pedigrees. As a result of the confidence in the KCNMA1 linkage to patient symptoms, paroxysmal nonkinesigenic dyskinesia-3 with or without generalized epilepsy (PNKD3; MIM #609446) is now recognized as distinct type of movement disorder linked to at least two mutations in KCNMA1 (D434G and N995S/N999S/N1053S).
For mutations where only a single patient defines the clinical presentation, causality must still be investigated. Nine additional de novo KCNMA1 mutations have been reported in the literature where a single patient represents the known symptomatic information. These mutations include: R458Ter*, G354S, S351Y, G356R, C413Y/N449fs, I6663V, P805L, E884K, and D98N (Zhang et al., 2015; Carvalho-de-Souza et al., 2016; Staisch et al., 2016; Liang et al., 2019; Fig. 1). In these cases, most of the symptoms are similar to those previously described and only a few symptoms do not overlap. For example, seven of the nine patients developed a movement disorder, two developed seizures, all nine were reported to have developmental delay, seven had intellectual disability, and four had abnormal brain imaging indicative of cerebellar atrophy. The new symptoms were mostly related to neurological dysfunction and included autistic like features and areflexia (Liang et al., 2019). These clinical findings suggest semi-distinct phenotypes associated with some of these mutations (Fig. 4), however the single patient sample size makes it difficult to validate this conclusion.
Lastly, three additional patients with epilepsy were identified to harbor other KCNMA1 mutations of unknown significance (K518N, E656A, and N1159S). While patch-clamp recordings classified these three variants as benign (Li et al., 2018), the shared symptom of epileptic seizure with other pathogenic KCNMA1 mutations may indicate these mutations interfere with BK activity and regulation of neuronal excitability through a yet-to-be-defined mechanism.
Across the KCNMA1-linked channelopathy patient population, another important outstanding question is whether patient symptoms and phenotypes can be classified with respect to the functional changes in BK channel activity produced by mutations. Patients with both GOF or LOF KNCMA1 mutations exhibit overlapping symptoms, especially with respect to seizures and neurodevelopment (Fig. 4). Thus, combined with the small patient sample size for each mutation, definitive categorization of specific symptoms with increases or decreases in BK channel activity is not yet possible. Moreover, published reports lack detailed clinical description, further limiting the ability to determine if particular symptoms are specific to GOF versus LOF channel mutations. Nevertheless, a few symptoms, primarily movement disorders and muscle dysfunction, appear to be somewhat distinct for GOF versus LOF mutations (Fig. 4). Below, we summarize the variability of symptoms pertaining to seizure, movement disorder, and neurodevelopment for all patients, while highlighting certain symptoms that may segregate with GOF or LOF mutations.
Movement disorder
A predominant phenotype of KCNMA1-linked channelopathy, regardless of the functional classification of the mutation (GOF, LOF, or VUS), is movement disorder. 24 of the 37 patients were reported to have symptoms related to a movement disorder with variability of the symptomatic presentation among patients. However, there seems to be more apparent segregation by channel functional classification for movement disorder compared with other predominant symptoms like seizures. These symptoms include PNKD (GOF) versus ataxia, axial hypotonia, and tremor (LOF; Fig. 4).
PNKD was reported in 17 of 20 GOF patients and only 2 of 13 LOF patients (Du et al., 2005; Zhang et al., 2015; Staisch et al., 2016; Wang et al., 2017; Yeşil et al., 2018; Heim et al., 2019). A patient with the VUS E884K mutation was also reported to have paroxysmal dyskinesia (Zhang et al., 2015). The paroxysmal dyskinesia symptoms were described as “nonkinesigenic” due to the lack of a movement trigger, in contrast to the more common paroxysmal kinesigenic dyskinesia, which is induced by various movements, and other forms of dyskinesia such as exertion-induced dyskinesia. Although the patients were diagnosed with PNKD, the specific types of movements and body parts involved during PNKD attacks were not stereotypical between patients. For example, the patients with D434G GOF mutation experienced episodes of involuntary mouth movements and hand stiffness during attacks (Du et al., 2005). Another patient harboring the G354S LOF mutation was reported to have perioral dyskinesia diagnosed at 18 mo (Staisch et al., 2016). One patient with the N1053S mutation was diagnosed with PNKD at 7 mo after having paroxysmal dystonic postures (Zhang et al., 2015). The three patients with N999S mutations were all diagnosed at different ages with alike paroxysmal drop attacks characterized by behavioral arrest and generalized dystonic posture or stiffening (Heim et al., 2019; A.L. Meredith and S. Keros, personal observations). The frequency and duration of theses movement attacks were variable, ranging from few to many dozens of attacks every day, and lasting a few seconds to minutes. OMIM defines this movement disorder phenotype as “PNKD type 3” or “PNKD3” (MIM #609446), because KCNMA1 was the third gene linked to a PNKD phenotype. The other two genes include MR1 located on chromosome 2q35 and PNKD2 on chromosome 2q31, and they correspond to PNKD1 (phenotype MIM #118800) and PNKD2 (phenotype MIM #611147), respectively. However, the specific term PKND3 is not systematically used for patients with PNKD and KCNMA1 mutations in the literature, suggesting existing and new patients expressing similar symptoms should be reevaluated for this diagnosis.
A movement disorder distinct from PNKD, and noted only in LOF patients, was ataxia (Fig. 4; Carvalho-de-Souza et al., 2016; Liang et al., 2019), a phenotype also well described in BK channel “knockout” mice harboring a LOF deletion in the KCNMA1 gene (Meredith et al., 2004; Sausbier et al., 2004; Chen et al., 2010). In patients, the ataxic symptoms included lack of coordination, unbalanced gait, and spasticity of lower extremities. Three of the five ataxic patients also had cerebellar atrophy diagnosed by brain MRI (Carvalho-de-Souza et al., 2016; Liang et al., 2019), which was another clinical finding distinct for LOF patients. Studies of KCNMA1−/− mouse models showed reduced basal firing from cerebellar Purkinje neurons and impaired cerebellar signaling and motor coordination, which may offer a possible mechanism for the ataxic symptoms in these patients (Sausbier et al., 2004). In addition, BK channel inhibitors produce ataxia when ingested or injected (Imlach et al., 2008; McManus and Rothberg, 2014; Hoshi and Heinemann, 2016; Kaczorowski and Garcia, 2016), supporting association between BK channel LOF and ataxic movement disorder.
Baseline axial hypotonia is another motor dysfunction symptom most prominent with LOF mutations (Fig. 4). The axial hypotonia reported in 8 of 13 LOF patients ranged from mild to severe and was independent from PNKD-associated symptoms noted in GOF patients (Tabarki et al., 2016;Liang et al., 2019). Additionally, tremor was noted in two LOF patients, and is a phenotype shared with BK channel KO mice (Sausbier et al., 2004; Imlach et al., 2008), but specific details describing this symptom were not provided (Yeşil et al., 2018; Liang et al., 2019). Further diagnostic studies are required to determine if these movement disorder attacks are due to mutant BK channel activity within the affected muscle groups, or whether they are secondary to CNS dysfunction, as both mechanisms are seen in a variety of channelopathies associated with movement disorders (Graves and Hanna, 2005).
Seizure disorder
Seizure is another predominant symptom associated with both GOF and LOF KCNMA1 mutations. 18 of the 37 patients described in this review developed epileptic seizures. However, there was considerable variability among the characteristics of the seizures such as age of onset, type, EEG pattern, frequency and duration, and response to medications. None of these characteristics are clearly delineated by the mutation type. Both GOF and LOF mutations are associated with overlapping seizure phenotypes including myoclonic (Tabarki et al., 2016; Li et al., 2018), generalized tonic-clonic (GTCS; Du et al., 2005; Tabarki et al., 2016; Yeşil et al., 2018), and absence seizures (Du et al., 2005; Li et al., 2018; Yeşil et al., 2018; Liang et al., 2019; Fig. 4). Interestingly, all nine GOF patients experienced absence seizures whereas only three of six LOF patients experienced them. Atonic seizures and status epilepticus were only reported in two different patients expressing LOF mutations (Yeşil et al., 2018; Liang et al., 2019). 10 of the 18 patients were reported to have abnormal inter-ictal EEG findings ranging from mild background slowing to generalized spike wave complexes to Lennox-Gastaut patterning (Du et al., 2005; Tabarki et al., 2016; Li et al., 2018; Yeşil et al., 2018). Age of onset for seizure also varied between patients regardless of the mutation type. Of the 13 family members expressing a D434G GOF mutation, 6 were diagnosed with epilepsy at ages ranging from 6 mo and 9 yr (Du et al., 2005). Three of the seven patients expressing a N995S or N999S GOF mutation began experiencing seizures at 20 mo, 6 yr, and 9 yr of age (Li et al., 2018; Heim et al., 2019; A.L. Meredith and S. Keros, personal observations), and the two siblings with the same Y676Lfs*7 putative LOF mutation had seizures beginning at one year of age (Tabarki et al., 2016). As in the general population, it is likely that seizure heterogeneity with this subset of patients is heavily influenced by additional factors (Steinlein, 2008).
Neurodevelopmental and cognitive phenotypes
Patients with both GOF and LOF KCNMA1 alleles showed developmental delay and intellectual disability (Fig. 4). Of the 37 patients reported in the literature, 21 were described as having developmental delay (Zhang et al., 2015; Carvalho-de-Souza et al., 2016; Wang et al., 2017; Li et al., 2018; Yeşil et al., 2018; Heim et al., 2019; Liang et al., 2019) and 12 were noted to have intellectual disability (Zhang et al., 2015; Carvalho-de-Souza et al., 2016; Wang et al., 2017; Heim et al., 2019; Liang et al., 2019). The developmental delays ranged from mild to severe and included psychomotor symptoms such as delayed/unstable sitting, inability to support head or walk unassisted, speech delay, and global developmental delay (Zhang et al., 2015; Tabarki et al., 2016; Heim et al., 2019; Liang et al., 2019). A subset of neurodevelopmental symptoms has only been described in patients with LOF KCNMA1 alleles (Fig. 4). One patient had hearing impairment (Liang et al., 2019), and multiple patients demonstrated visual impairment due to nystagmus, strabismus, and macular coloboma (Carvalho-de-Souza et al., 2016; Liang et al., 2019). Two patients had autism disorder or features (Heim et al., 2019; A.L. Meredith and S. Keros, personal observations), which together with mental retardation was previously associated with a potential LOF SNP in KCNMA1 (Laumonnier et al., 2006).
Lessons from animal studies linking changes in BK channel function to neurological phenotypes
Within the rodent brain, high levels of BK channel expression are detected in the neocortex, basal ganglia, hippocampus, thalamus, habenula and its tract to the interpeduncular nucleus in the midbrain, cerebellum, vestibular nuclei in the hindbrain, and olfactory system (Knaus et al., 1996; Wanner et al., 1999; Misonou et al., 2006; Sausbier et al., 2006b; Yue et al., 2014). Animals with functional deficiency of BK channels displayed behaviors that can recapitulate some of the movement symptoms in patients with putative LOF KCNMA1 mutations. Pharmacological inhibition of BK channels with lolitrem B or paxilline induced tremor and ataxia in mice and livestock (Imlach et al., 2008), and constitutive deletion of KCNMA1 (KCNMA1−/−) produced several types of motor impairments such as ataxia, tremor, impaired motor coordination, reduced muscle strength, and abnormal eye blink reflex in mice (Meredith et al., 2004; Sausbier et al., 2004; Imlach et al., 2008; Typlt et al., 2013a). The motor impairments exhibited by KCNMA1−/− mice suggested cerebellar dysfunction. Consistent with this, KCNMA1−/− Purkinje neurons showed significantly reduced basal firing activity and increased short term transmission depression at synapses on deep cerebellar nuclei and disrupted olivo-cerebellar feedback (Sausbier et al., 2004; Chen et al., 2010). The direct influence of GOF mutations in BK channel function on motor control has not yet been investigated, but mice harboring a strong (nonpatient-derived) GOF mutation that increases voltage-dependent gating of the BK channel (R207Q) have grossly normal locomotor behavior (Montgomery and Meredith, 2012). Taken together, the results from animal models demonstrate that BK channel LOF is associated with movement disorder phenotypes that are also observed in human patients, and further suggest the potential for segregation of distinct motor dysfunction with LOF versus GOF BK channel alterations.
Unlike motor dysfunction, both LOF and GOF alterations in BK channel activity have been linked with the seizure pathophysiology in animal studies. In a chronic temporal lobe epileptic rat model, expression of the BK channel α-subunit encoded by KCNMA1 was down-regulated in hippocampus and cortex (Ermolinsky et al., 2008; Pacheco Otalora et al., 2008), suggesting a link between seizure and LOF in BK channel activity. BK channel expression and BK current levels were also reduced in the inferior colliculus during alcohol withdrawal periods in a rat model of alcohol withdrawal seizures (N’Gouemo et al., 2009). However, a direct causal relationship between LOF alterations in BK channel activity and epileptogenesis has not yet been reported.
There is a more solid correlation between GOF alterations in BK channel activity and seizure. Deletion of regulatory β4 subunit (KCNMB4−/−), which slows BK channel activation, produced temporal lobe seizures cortex in mice, associated with hyperactive firing in dentate granule (DG) cells in the hippocampus (Brenner et al., 2005). In addition, kainic acid–induced seizure susceptibility was increased in β4 heterozygous mice (Whitmire et al., 2017). Furthermore, in a model of fragile X syndrome, enhancement of BK channel activity in KCNMB4−/− mice was associated with alterations in epileptiform activity in hippocampal slices (Deng and Klyachko, 2016). Several other studies also show situations where increased BK channel expression was associated with hyperexcitability and seizure activity. Elevated cell surface expression and activity of BK channels via mutation of CRL4ACRBN ubiquitin ligase increased susceptibility to spontaneous and PTZ-induced seizures in mice, an effect that can be reduced by pretreatment of the mice with the BK channel inhibitor paxilline (Liu et al., 2014). Up-regulation of BK α and down-regulation of β4 protein were reported after seizure induction in rats (Savina et al., 2014), and β4 mRNA was down-regulated in DG cells in the hippocampus following pilocarpine-induced seizure (Whitmire et al., 2017). BK currents in somatosensory cortex were significantly increased after picrotoxin-induced seizure in mice, and the increased neuronal firing activity was normalized with BK channel inhibitors (Shruti et al., 2008). Similarly, BK blockers normalized DG cell firing rates that were increased during pilocarpine-induced seizure episodes in rats (Mehranfardet al., 2014, 2015). These animal studies show that GOF alterations in BK channel activity, either via increased expression or due to loss of the slow gating imparted by the β4 subunit, alters the balance of neuronal activity in the brain. GOF KCNMA1 mutations affecting BK channel activity in mechanistically similar ways could be causative in KCNMA1-linked patient seizures.
Inheritance and prevalence of KCNMA1-linked channelopathy
The majority of the pathogenic KCNMA1 mutations reported in the literature occurred de novo, however few mutations exhibit autosomal recessive or autosomal dominant inheritance patterns (Fig. 1). To date, the literature reports a total of 16 mutations identified in 37 symptomatic patients (Du et al., 2005; Zhang et al., 2015; Carvalho-de-Souza et al., 2016; Tabarki et al., 2016; Wang et al., 2017; Li et al., 2018; Yeşil et al., 2018; Heim et al., 2019; Liang et al., 2019). 17 patients have de novo mutations, which constitute 9/16 KCNMA1 mutations. 4 patients inherited an autosomal recessive mutation, constituting 3/16 mutations. 13 patients (one family) inherited a single (1/16) autosomal dominant mutation. Additionally, there are three benign KCNMA1 genetic variants (3/16) identified in three patients with epilepsy (Li et al., 2018). One of these three variants exhibited an autosomal recessive inheritance pattern, but the inheritance patterns for the other two variants were not determined.
KCNMA1-linked channelopathy is a very rare syndrome. The allele frequency of certain pathogenic KCNMA1 mutations in larger populations is estimated to be less than 1:100,000 in the Genome Aggregation Database (D434G = 3 × 10−5 and Y676Lfs*7 = 4 × 10−6; Lek et al., 2016). However, the allele frequency in larger populations is unknown for majority of the pathogenic KCNMA1 mutations owing to the rarity of this syndrome. Table S1 provides information regarding the number of missense SNPs present in KCNMA1 and putative pathogenic mutations reported by different large-scale sequencing databases.
Diagnosis and treatment of KCNMA1-linked channelopathy
Epilepsy and movement disorders are known to be caused by several different medical conditions, a subset of which are the result of monogenetic channelopathies (Brenner and Wilcox, 2012; Kim, 2014). Traditionally, if a patient presents with epileptic seizures and movement attacks with otherwise unremarkable brain imaging and normal neurological function between episodes, then a genetic channelopathy is a leading consideration and genetic testing is recommended to search for causative genetic mutations. The D434G mutation was initially mapped via microsatellite linkage analysis (Du et al., 2005); however, causative genetic findings in neurological disorders are more routinely established now by single gene analysis, panels of multiple gene tests for disorders which share a specific phenotype, or by high-powered WES or whole genome sequencing (WGS; Pulst, 1999). When patient symptoms are extensive and a test for multiple pathogenic genetic mutations is desired, WES is typically used instead of WGS (Kong et al., 2018). WES is less costly than WGS, and the majority of pathogenic variants are found within the coding regions or splice sites of proteins. However, WES is prone to incomplete coverage of certain loci, variability in coverage from person to person, and has susceptibility to false positives and negatives. Of the 37 patients described in the literature, 21 were identified to have a KCNMA1 mutation using WES (Staisch et al., 2016; Tabarki et al., 2016; Li et al., 2018; Yeşil et al., 2018; Heim et al., 2019; Liang et al., 2019).
On the other hand, when the clinical symptoms are well defined or stereotypical such as generalized epilepsy and paroxysmal dyskinesia, as is the case with some patients with KCNMA1-linked channelopathy, a limited set of specific candidate genes are assayed. At present, 29 commercial gene panels include KCNMA1 (Table S2). Of note, the genes included with each panel are frequently updated as additional genetic mutations are associated with specific medical conditions. Three of the 37 patients with KCNMA1 mutations were identified using epilepsy- and paroxysmal dyskinesia–specific gene panels (Zhang et al., 2015;Wang et al., 2017).
Development of treatment regimens specific for KCNMA1-linked channelopathy (“precision medicine”) is currently limited by several important factors, including (a) lack of FDA-approved selective BK channel pharmacological modulators, (b) lack of stereotypical clinical presentations with respect to core symptoms (movement disorder and seizure), (c) lack of established causality between alterations in channel activity and patient symptoms, and (d) inadequate medical assessment identifying the loci for symptoms in patients. Thus, the majority KCNMA1-linked channelopathy patients with seizures have been treated with anti-seizure medications (ASMs) selected with the same general approach as for any nonsyndromic epilepsy. Several KCNMA1 patient studies commented on the efficacy of various ASMs and demonstrate the range of patient responses may range from positive, to no response, to negative.
Sodium valproate and/or lamotrigine were effective in treating the absence seizures in the proband with the GOF D434G mutation (Du et al., 2005), as well as the absence, GTCS, and atonic seizures in a patient with the LOF R458Ter* mutation (Yeşil et al., 2018), and myoclonic seizures in a patient with homozygous LOF Tyr676Leufs*7 mutation (Tabarki et al., 2016). These two ASMs were also used to treat absence seizures in two patients with the LOF G375R mutation, but efficacy of the treatments was not reported (Liang et al., 2019). The second patient with the homozygous Tyr676Leufs*7 mutation and myoclonic seizures and GTCS was treated effectively with sodium valproate and levetiracetam (Tabarki et al., 2016). Levetiracetam alone was effective to treat atypical absence and myoclonic seizures in two patients with the GOF N995S mutation (Li et al., 2018). However, one of these patients was trialed on various ASMs before levetiracetam; treatment with oxcarbazepine, and sodium valproate produced negative side effects, sultiame and perampanel did not have any effects, and zonisamide monotherapy reduced the seizure frequency, but failed to stop the myoclonic seizures. Notably, this patient experienced significant worsening of seizures with ethosuxmide, the drug of choice for the absence seizures seen in childhood absence epilepsy. A dramatic worsening of absence seizures at very low doses of ethosuximide also occurred in a GOF N999S patient, reported in Heim et al. (2019), while levetiracetam was not tolerated due to side effects (S. Keros, personal observation). The patient with a LOF D984N mutation was diagnosed with status epilepticus and treated with several ASMs including clobazam, ethosuximide, lamotrigine, zonisamide, sodium valproate, phenobarbital, and lacosamide, but the therapeutic efficacy was not known (Liang et al., 2019).
Paroxysmal dyskinesias are commonly categorized into three main types (kinesigenic or paroxysmal kinesigenic dyskinesia, nonkinesigenic or PNKD, and exercise-induced or PED), and each type has been shown to respond differently to medications (Unterberger and Trinka, 2008). PNKD, the dominant type associated with KCNMA1-linked channelopathy, is commonly described as refractory to medications. However, benzodiazepines, particularly clonazepam, and some ASMs have been shown to be effective in some PNKD patients (Unterberger and Trinka, 2008). Additional therapies used to treat other movement disorders, such as acetazolamide in episodic ataxia, have demonstrated positive effects in some patients with PNKD (Silveira-Moriyama et al., 2018). Acetazolamide is a carbonic anhydrase inhibitor and has been suggested as a possible direct agonist of BK channels in rat cerebellar neurons and skeletal muscle (Tricarico et al., 2004; Abbasi et al., 2014; Tricarico and Mele, 2017). However, the potentiating mechanism for acetazolamide on either WT or mutant BK channels is not currently well established.
There are a limited number of reports assessing PNKD treatment and related symptoms in patients harboring KCNMA1 mutations. Clonazepam was partially effective for two patients with GOF D434G mutations (Du et al., 2005) and reduced the frequency of PNKD attacks in one patient with the N1053S mutation (Wang et al., 2017). Clonazepam was also used to treat dyskinetic tremor, dystonia, and spasticity of the lower extremities in a patient with the R458Ter* mutation (Yeşil et al., 2018). A second patient with the GOF N1053S mutation and paroxysmal dystonic postures was unresponsive to oxcarbazepine, sodium valproate, and levetiracetam (Zhang et al., 2015), whereas a patient with the GOF N999S mutation experiencing paroxysmal drop attacks responded positively to DHA and acetazolamide after not responding to clonazepam, amitriptyline, or levetiracetam (Heim et al., 2019). A second patient with the N999S mutation and experiencing the paroxysmal drop attacks did not respond to levetiracetam, carbamazepine, valproic acid, vitamin B complex, CBD oil, DHA, 5-HIAA, or pimozide (Heim et al., 2019; A.L. Meredith and S. Keros, personal observations). Overall, within the KCNMA1-linked channelopathy population, patient responses to therapies treating seizure and movement disorders are not predictable a priori.
Precision medicine and future directions
For most genetic channelopathies, pharmacologic treatment does not yet consist of modulating the specific activity of mutant ion channels. For KCNMA1 and other channelopathies, the first hurdle to precision medicine involves categorizing patient mutations into functional classes, such as GOF or LOF. However, these classes are not trivial to define and can be condition- or cell-type specific, related to the particular splice variants, accessory subunits, post-translational channel modifications, and cellular contexts (intracellular calcium, in particular for the BK channel) in which the mutation is tested. For this reason, it is not yet clear whether strategies using patient-derived stem cells, which can only be differentiated into a limited set of cell types, would result in BK channel pharmacological modulators that are ultimately clinically relevant. In addition, GOF, LOF, and potentially benign mutations in BK channel activity are associated with overlapping symptoms (Fig. 4), raising the question of whether selective agonists or antagonists that restore the “correct” level of BK channel activity will actually produce the desired outcome on neuronal activity. Without more detailed information about the origination of the changes in excitability underlying the seizure and movement symptoms in KCNMA1-linked channelopathy, it is difficult to ascertain which BK channel components to target, and in which neuron or muscle loci.
Selective BK channel pharmacotherapy has been under development for over two decades, mostly intended to treat smooth muscle dysfunction resulting from LOF in BK channel activity (hypertension, bladder incontinence, stroke, and erectile dysfunction; Christ et al., 2001; Gribkoff et al., 2001; Spektor et al., 2002; Vang et al., 2010; Soder and Petkov, 2011; Liu et al., 2013). Several compounds, including endogenous, naturally occurring, and synthetic, have been identified as BK agonists and trialed as potential therapeutic agents (Hou et al., 2009; Bentzen et al., 2014; Hoshi and Heinemann, 2016). The endogenous class includes heme and heme-breakdown products (Tang et al., 2003; Horrigan et al., 2005), free long-chain polyunsaturated acids (Clarke et al., 2002, 2003), metabolites of cytochrome P450, epoxygenase and lipoxygenase (Félétou, 2009; Hou et al., 2009), and 17 β-estradiol (Valverde et al., 1999). The natural occurring class includes a variety of entities found in herbs, roots, and leaves used in folk medicines for treating asthma and other disorders stemming from smooth muscle dysfunction such as DHS-I (McManus et al., 1993; Nardi et al., 2003). The synthetic class includes benzimidazolone compounds NS004 and NS1619 (Olesen et al., 1994a,b), the more potent and selective NS11021 (Bentzen et al., 2007), and a newer family of BK channel activators called the GoSlo-SR family (anthra-quinone analogues; Roy et al., 2012). At least one compound, Cym04 (a dehydroabietic acid derivative) has been shown to demonstrate some specificity for a particular BK channel splice variant (Cui et al., 2008; Gessner et al., 2012). Despite the ability of these chemical agents to activate BK channels in vitro and animal studies, only a single drug with therapeutic action targeting the BK channel is FDA approved (Cuppoletti et al., 2007). This drug, Rescula (unoprostone isopropyl ophthalmical solution) is a potent BK channel activator used to reduce ocular pressure in glaucoma (Thieme et al., 2001).
Other potential, but nonselective, neuromodulators that may affect BK channels include docosahexaenoic acid (DHA), an omega-3 fatty acid found in oily fish, acetazolamide, zonisamide, and riluzole. DHA reversibly binds BKα/β1 expressed primarily in smooth muscle and BKα/β4 channels expressed primarily in the nervous system in mice. DHA increases peak BK current by 20- to 30-fold at certain physiological voltages (Hoshi et al., 2013a), suggesting potential efficacy for enhancing LOF BK channel activity. While DHA injection in mice lowers blood pressure (Hoshi et al., 2013b), the physiological roles of this fatty acid within the nervous system are not well defined. Injection of DHA in rats increased latency of PTZ-induced seizure implicating its neuroprotective effect ((Trépanier et al., 2014)); however, a systemic review assessing protective role of omega-3 supplementation in human seizures reported inconclusive evidence of benefit (Pourmasoumi et al., 2018). Two patients with KCNMA1-linked channelopathy have been trialed on DHA (in combination with acetazolamide; Heim et al., 2019). However, the patients harbor a mutation classified as GOF, suggesting any potential therapeutic effect conferred by DHA might not originate from direct modulation of the BK channel. Thus, the data provide no specific indication on the therapeutic potential for DHA in KCNMA1-linked channelopathy patients. Similarly, although acetazolamide has been reported to have agonist effects on BK channels in skeletal muscle (Tricarico et al., 2004; Dinardo et al., 2012), this has not yet been substantiated on BK channels expressed in heterologous cells or neurons. Two other neurological drugs, zonisamide (an ASM) and riluzole, increased K+ currents in hippocampal and skeletal cell lines respectively, an effect that was blocked by BK channel inhibitors (Huang et al., 2007; Wang et al., 2008).
Another potential pharmacotherapy based on FDA-approved drugs to normalize BK channel activity associated with GOF mutations is Ca2+ channel inhibitors. For GOF mutations, decreasing the Ca2+-dependent activation could reduce BK current. While this approach could require knowledge of the specific Ca2+ source for the channels in tissues where excitability is altered in the patients, one class of inhibitors, dihydropyridines (DHPs), are known to inhibit BK channel activation in a variety of central neurons (Fagni et al., 1994; Zhang and Gold, 2009; Wang et al., 2016; Whitt et al., 2018). The DHPs, nifedipine and nimodipine, and the non-DHP verapamil, are used for some types of dyskinesia (Abad and Ovsiew, 1993). Verapamil has been used for refractory epilepsy and hyperkinetic movement disorders (Ovsiew et al., 1998; Narayanan et al., 2016; Lakshmikanthcharan et al., 2018). In addition, there is at least one report that Verapamil might block BK channels directly, further contributing to a reduction in channel activity through a selective mechanism (Harper et al., 2001). However, it has not yet been investigated whether doses effective at reducing BK channel activity in the brain would have cardiac side effects.
Beyond pharmacology, some therapeutic approaches tailored for individual patients with other monogenetic disorders may be conceivable in the future for KCNMA1-linked channelopathy, such as gene editing, gene therapy, or optogenetic restoration of neuronal activity. Yet, substantial challenges are present for their application to any disease or disorder, including target specificity, delivery optimization, elimination of off-target side effects, timing optimization before or after critical stages of neurological development (Wykes and Lignani, 2018), and creating animal models that best replicate the genetic channelopathy from the molecular level to the clinical phenotype to determine initial safety and efficacy of such therapies (Tanner and Beeton, 2018). While these precision medicines may hold eventual promise, until the challenges are overcome, development of additional pharmacological approaches remains the major path forward clinically.
Supplementary Material
Acknowledgments
Sharona E. Gordon served as editor.
We thank Jenna Harvey for preliminary review of the human KCNMA1 mutations. Figure 1 was created with BioRender.com.
This work was supported by grants from the National Heart, Lung, and Blood Institute (R01-HL102758 to A.L. Meredith), the Training Program in Integrative Membrane Biology, National Heart, Lung, and Blood Institute (T32-GM008181 to A.L. Meredith), the American Physiological Society’s Ryuji Ueno award, sponsored by the S & R Foundation (to A.L. Meredith), and a University of Maryland School of Medicine Proposed Research Initiated by Students and Mentors (PRISM) Program Merit Award (to C.S. Bailey).
The authors declare no competing financial interests.
References
- Abad V., and Ovsiew F.. 1993. Treatment of persistent myoclonic tardive dystonia with verapamil Br. J. Psychiatry. 162:554–556. 10.1192/bjp.162.4.554 [DOI] [PubMed] [Google Scholar]
- Abbasi S., Abbasi A., and Sarbaz Y.. 2014. Introducing treatment strategy for cerebellar ataxia in mutant med mice: combination of acetazolamide and 4-aminopyridine. Comput. Methods Programs Biomed. 113:697–704. 10.1016/j.cmpb.2013.11.008 [DOI] [PubMed] [Google Scholar]
- Adams P.R., Constanti A., Brown D.A., and Clark R.B.. 1982. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 296:746–749. 10.1038/296746a0 [DOI] [PubMed] [Google Scholar]
- Becker M.N., Brenner R., and Atkinson N.S.. 1995. Tissue-specific expression of a Drosophila calcium-activated potassium channel. J. Neurosci. 15:6250–6259. 10.1523/JNEUROSCI.15-09-06250.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell T.J., Miyashiro K.Y., Sul J.Y., McCullough R., Buckley P.T., Jochems J., Meaney D.F., Haydon P., Cantor C., Parsons T.D., and Eberwine J.. 2008. Cytoplasmic BKCa channel intron-containing mRNAs contribute to the intrinsic excitability of hippocampal neurons. Proc. Natl. Acad. Sci. USA. 105:1901–1906. 10.1073/pnas.0711796105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton M.D., Lewis A.H., Bant J.S., and Raman I.M.. 2013. Iberiotoxin-sensitive and -insensitive BK currents in Purkinje neuron somata. J. Neurophysiol. 109:2528–2541. 10.1152/jn.00127.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzen B.H., Nardi A., Calloe K., Madsen L.S., Olesen S.P., and Grunnet M.. 2007. The small molecule NS11021 is a potent and specific activator of Ca2+-activated big-conductance K+ channels. Mol. Pharmacol. 72:1033–1044. 10.1124/mol.107.038331 [DOI] [PubMed] [Google Scholar]
- Bentzen B.H., Olesen S.P., Rønn L.C., and Grunnet M.. 2014. BK channel activators and their therapeutic perspectives. Front. Physiol. 5:389 10.3389/fphys.2014.00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkefeld H., and Fakler B.. 2008. Repolarizing responses of BKCa-Cav complexes are distinctly shaped by their Cav subunits. J. Neurosci. 28:8238–8245. 10.1523/JNEUROSCI.2274-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock T., and Stuart G.J.. 2016. The Impact of BK Channels on Cellular Excitability Depends on their Subcellular Location. Front. Cell. Neurosci. 10:206 10.3389/fncel.2016.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R., and Wilcox K.S.. 2012. Potassium Channelopathies of Epilpesy. In Jasper’s Basic Mechanisms of the Epilepsies. Noebels J.L., Avoli M., Rogawski M.A., Olsen R.W., and Delgado-Escueta A.V., editors. 1029–1048. [Google Scholar]
- Brenner R., Jegla T.J., Wickenden A., Liu Y., and Aldrich R.W.. 2000. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J. Biol. Chem. 275:6453–6461. 10.1074/jbc.275.9.6453 [DOI] [PubMed] [Google Scholar]
- Brenner R., Chen Q.H., Vilaythong A., Toney G.M., Noebels J.L., and Aldrich R.W.. 2005. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat. Neurosci. 8:1752–1759. 10.1038/nn1573 [DOI] [PubMed] [Google Scholar]
- Brown S.M., Bentcheva-Petkova L.M., Liu L., Hristov K.L., Chen M., Kellett W.F., Meredith A.L., Aldrich R.W., Nelson M.T., and Petkov G.V.. 2008. Beta-adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am. J. Physiol. Renal Physiol. 295:F1149–F1157. 10.1152/ajprenal.00440.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-de-Souza J., Kubota T., Du X., Latorre R., Gomez C.M., and Bezanilla F.. 2016. A Missense Mutation in the Selectivity Filter of BK Affects the Channel’s Potassium Conductance. Biophys. J. 110:499a 10.1016/j.bpj.2015.11.2412 [DOI] [Google Scholar]
- Chen X., Kovalchuk Y., Adelsberger H., Henning H.A., Sausbier M., Wietzorrek G., Ruth P., Yarom Y., and Konnerth A.. 2010. Disruption of the olivo-cerebellar circuit by Purkinje neuron-specific ablation of BK channels. Proc. Natl. Acad. Sci. USA. 107:12323–12328. 10.1073/pnas.1001745107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ G.J., Day N.S., Day M., Santizo C., Zhao W., Sclafani T., Zinman J., Hsieh K., Venkateswarlu K., Valcic M., and Melman A.. 2001. Bladder injection of “naked” hSlo/pcDNA3 ameliorates detrusor hyperactivity in obstructed rats in vivo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281:R1699–R1709. 10.1152/ajpregu.2001.281.5.R1699 [DOI] [PubMed] [Google Scholar]
- Christ G.J., Andersson K.E., Williams K., Zhao W., D’Agostino R. Jr., Kaplan J., Aboushwareb T., Yoo J., Calenda G., Davies K.P., et al. . 2009. Smooth-muscle-specific gene transfer with the human maxi-k channel improves erectile function and enhances sexual behavior in atherosclerotic cynomolgus monkeys. Eur. Urol. 56:1055–1066. 10.1016/j.eururo.2008.12.016 [DOI] [PubMed] [Google Scholar]
- Clarke A.L., Petrou S., Walsh J.V. Jr, and Singer J.J.. 2002. Modulation of BKCa channel activity by fatty acids: structural requirements and mechanism of action. Am. J. Physiol. Cell Physiol. 283:C1441–C1453. 10.1152/ajpcell.00035.2002 [DOI] [PubMed] [Google Scholar]
- Clarke A.L., Petrou S., Walsh J.V. Jr., and Singer J.J.. 2003. Site of action of fatty acids and other charged lipids on BKCa channels from arterial smooth muscle cells. Am. J. Physiol. Cell Physiol. 284:C607–C619. 10.1152/ajpcell.00364.2002 [DOI] [PubMed] [Google Scholar]
- Contet C., Goulding S.P., Kuljis D.A., and Barth A.L.. 2016. BK Channels in the Central Nervous System. Int. Rev. Neurobiol. 128:281–342. 10.1016/bs.irn.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Yang H., and Lee U.S.. 2009. Molecular mechanisms of BK channel activation. Cell. Mol. Life Sci. 66:852–875. 10.1007/s00018-008-8609-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y.M., Yasutomi E., Otani Y., Yoshinaga T., Ido K., Sawada K., and Ohwada T.. 2008. Novel BK channel openers containing dehydroabietic acid skeleton: structure-activity relationship for peripheral substituents on ring C. Bioorg. Med. Chem. Lett. 18:5201–5205. 10.1016/j.bmcl.2008.08.078 [DOI] [PubMed] [Google Scholar]
- Cuppoletti J., Malinowska D.H., Tewari K.P., Chakrabarti J., and Ueno R.. 2007. Cellular and molecular effects of unoprostone as a BK channel activator. Biochim. Biophys. Acta. 1768:1083–1092. 10.1016/j.bbamem.2006.12.015 [DOI] [PubMed] [Google Scholar]
- Deng P.Y., and Klyachko V.A.. 2016. Genetic upregulation of BK channel activity normalizes multiple synaptic and circuit defects in a mouse model of fragile X syndrome. J. Physiol. 594:83–97. 10.1113/JP271031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez-Sampedro A., Silverman W.R., Bautista J.F., and Richerson G.B.. 2006. Mechanism of increased open probability by a mutation of the BK channel. J. Neurophysiol. 96:1507–1516. 10.1152/jn.00461.2006 [DOI] [PubMed] [Google Scholar]
- Dinardo M.M., Camerino G., Mele A., Latorre R., Conte Camerino D., and Tricarico D.. 2012. Splicing of the rSlo gene affects the molecular composition and drug response of Ca2+-activated K+ channels in skeletal muscle. PLoS One. 7:e40235 10.1371/journal.pone.0040235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Bautista J.F., Yang H., Diez-Sampedro A., You S.A., Wang L., Kotagal P., Lüders H.O., Shi J., Cui J., et al. . 2005. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat. Genet. 37:733–738. 10.1038/ng1585 [DOI] [PubMed] [Google Scholar]
- Düfer M., Neye Y., Hörth K., Krippeit-Drews P., Hennige A., Widmer H., McClafferty H., Shipston M.J., Häring H.U., Ruth P., and Drews G.. 2011. BK channels affect glucose homeostasis and cell viability of murine pancreatic beta cells. Diabetologia. 54:423–432. 10.1007/s00125-010-1936-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworetzky S.I., Trojnacki J.T., and Gribkoff V.K.. 1994. Cloning and expression of a human large-conductance calcium-activated potassium channel. Brain Res. Mol. Brain Res. 27:189–193. 10.1016/0169-328X(94)90203-8 [DOI] [PubMed] [Google Scholar]
- Ermolinsky B., Arshadmansab M.F., Pacheco Otalora L.F., Zarei M.M., and Garrido-Sanabria E.R.. 2008. Deficit of Kcnma1 mRNA expression in the dentate gyrus of epileptic rats. Neuroreport. 19:1291–1294. 10.1097/WNR.0b013e3283094bb6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essin K., Gollasch M., Rolle S., Weissgerber P., Sausbier M., Bohn E., Autenrieth I.B., Ruth P., Luft F.C., Nauseef W.M., and Kettritz R.. 2009. BK channels in innate immune functions of neutrophils and macrophages. Blood. 113:1326–1331. 10.1182/blood-2008-07-166660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber E.S., and Sah P.. 2002. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J. Neurosci. 22:1618–1628. 10.1523/JNEUROSCI.22-05-01618.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber E.S., and Sah P.. 2003. Calcium-activated potassium channels: multiple contributions to neuronal function. Neuroscientist. 9:181–194. 10.1177/1073858403009003011 [DOI] [PubMed] [Google Scholar]
- Fagerberg L., Hallström B.M., Oksvold P., Kampf C., Djureinovic D., Odeberg J., Habuka M., Tahmasebpoor S., Danielsson A., Edlund K., et al. . 2014. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteomics. 13:397–406. 10.1074/mcp.M113.035600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagni L., Bossu J.L., and Bockaert J.. 1994. Inhibitory effects of dihydropyridines on macroscopic K+ currents and on the large-conductance Ca2+-activated K+ channel in cultured cerebellar granule cells. Pflugers Arch. 429:176–182. 10.1007/BF00374310 [DOI] [PubMed] [Google Scholar]
- Fakler B., and Adelman J.P.. 2008. Control of KCa channels by calcium nano/microdomains. Neuron. 59:873–881. 10.1016/j.neuron.2008.09.001 [DOI] [PubMed] [Google Scholar]
- Félétou M. 2009. Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br. J. Pharmacol. 156:545–562. 10.1111/j.1476-5381.2009.00052.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa J.A., Bonev A.D., Straub S.V., Meredith A.L., Wilkerson M.K., Aldrich R.W., and Nelson M.T.. 2006. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci. 9:1397–1403. 10.1038/nn1779 [DOI] [PubMed] [Google Scholar]
- Gessner G., Cui Y.M., Otani Y., Ohwada T., Soom M., Hoshi T., and Heinemann S.H.. 2012. Molecular mechanism of pharmacological activation of BK channels. Proc. Natl. Acad. Sci. USA. 109:3552–3557. 10.1073/pnas.1114321109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H., Bonev A.D., Hannah R.M., Meredith A., Aldrich R.W., and Nelson M.T.. 2010. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc. Natl. Acad. Sci. USA. 107:3811–3816. 10.1073/pnas.0914722107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis A.H., Moghadam S.H., and du Lac S.. 2010. Mechanisms of sustained high firing rates in two classes of vestibular nucleus neurons: differential contributions of resurgent Na, Kv3, and BK currents. J. Neurophysiol. 104:1625–1634. 10.1152/jn.00378.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldklang M.P., Perez-Zoghbi J.F., Trischler J., Nkyimbeng T., Zakharov S.I., Shiomi T., Zelonina T., Marks A.R., D’Armiento J.M., and Marx S.O.. 2013. Treatment of experimental asthma using a single small molecule with anti-inflammatory and BK channel-activating properties. FASEB J. 27:4975–4986. 10.1096/fj.13-235176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollasch M., Tank J., Luft F.C., Jordan J., Maass P., Krasko C., Sharma A.M., Busjahn A., and Bähring S.. 2002. The BK channel beta1 subunit gene is associated with human baroreflex and blood pressure regulation. J. Hypertens. 20:927–933. 10.1097/00004872-200205000-00028 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez V., and Lingle C.J.. 2019. Regulation of BK Channels by Beta and Gamma Subunits. Annu. Rev. Physiol. 81:113–137. 10.1146/annurev-physiol-022516-034038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves T.D., and Hanna M.G.. 2005. Neurological channelopathies. Postgrad. Med. J. 81:20–32. 10.1136/pgmj.2004.022012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribkoff V.K., Starrett J.E. Jr., Dworetzky S.I., Hewawasam P., Boissard C.G., Cook D.A., Frantz S.W., Heman K., Hibbard J.R., Huston K., et al. . 2001. Targeting acute ischemic stroke with a calcium-sensitive opener of maxi-K potassium channels. Nat. Med. 7:471–477. 10.1038/86546 [DOI] [PubMed] [Google Scholar]
- Grimes W.N., Li W., Chávez A.E., and Diamond J.S.. 2009. BK channels modulate pre- and postsynaptic signaling at reciprocal synapses in retina. Nat. Neurosci. 12:585–592. 10.1038/nn.2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm P.R., and Sansom S.C.. 2010. BK channels and a new form of hypertension. Kidney Int. 78:956–962. 10.1038/ki.2010.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N., Vervaeke K., and Storm J.F.. 2007. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J. Physiol. 580:859–882. 10.1113/jphysiol.2006.126367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halm S.T., Bottomley M.A., Almutairi M.M., Di Fulvio M., and Halm D.R.. 2017. Survival and growth of C57BL/6J mice lacking the BK channel, Kcnma1: lower adult body weight occurs together with higher body fat. Physiol. Rep. 5:5 10.14814/phy2.13137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Yang B.Z., Kranzler H.R., Liu X., Zhao H., Farrer L.A., Boerwinkle E., Potash J.B., and Gelernter J.. 2013. Integrating GWASs and human protein interaction networks identifies a gene subnetwork underlying alcohol dependence. Am. J. Hum. Genet. 93:1027–1034. 10.1016/j.ajhg.2013.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper A.A., Catacuzzeno L., Trequattrini C., Petris A., and Franciolini F.. 2001. Verapamil block of large-conductance Ca-activated K channels in rat aortic myocytes. J. Membr. Biol. 179:103–111. 10.1007/s002320010041 [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Morinaga S., Zhang J., Satoh Y., Meredith A.L., Nakata T., Wu Z., Kohsaka S., Inoue K., and Nakanishi H.. 2016. BK channels in microglia are required for morphine-induced hyperalgesia. Nat. Commun. 7:11697 10.1038/ncomms11697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hei H., Gao J., Dong J., Tao J., Tian L., Pan W., Wang H., and Zhang X.. 2016. BK Knockout by TALEN-Mediated Gene Targeting in Osteoblasts: KCNMA1 Determines the Proliferation and Differentiation of Osteoblasts. Mol. Cells. 39:530–535. 10.14348/molcells.2016.0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim J., Vemuri A., Lewis S., Meredith A., Keros S., and Kruer M.. 2019. Drop attacks in patients with KCNMA1 p.N999S heterozygous de novo mutations. 6th International Symposium on Paediatric Movement Disorders.
- Henne J., and Jeserich G.. 2004. Maturation of spiking activity in trout retinal ganglion cells coincides with upregulation of Kv3.1- and BK-related potassium channels. J. Neurosci. Res. 75:44–54. 10.1002/jnr.10830 [DOI] [PubMed] [Google Scholar]
- Hille B. 2001. Ion Channels of Excitable Membranes. Sinauer Associates, Sunderland, MA. [Google Scholar]
- Hirukawa K., Muraki K., Ohya S., Imaizumi Y., and Togari A.. 2008. Electrophysiological properties of a novel Ca2+-activated K+ channel expressed in human osteoblasts. Calcif. Tissue Int. 83:222–229. 10.1007/s00223-008-9167-9 [DOI] [PubMed] [Google Scholar]
- Horrigan F.T., and Aldrich R.W.. 2002. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J. Gen. Physiol. 120:267–305. 10.1085/jgp.20028605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan F.T., Cui J., and Aldrich R.W.. 1999. Allosteric voltage gating of potassium channels I. Mslo ionic currents in the absence of Ca2+ J. Gen. Physiol. 114:277–304. 10.1085/jgp.114.2.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan F.T., Heinemann S.H., and Hoshi T.. 2005. Heme regulates allosteric activation of the Slo1 BK channel J. Gen. Physiol. 126:7–21. 10.1085/jgp.200509262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., and Heinemann S.H.. 2016. Modulation of BK Channels by Small Endogenous Molecules and Pharmaceutical Channel Openers. Int. Rev. Neurobiol. 128:193–237. 10.1016/bs.irn.2016.03.020 [DOI] [PubMed] [Google Scholar]
- Hoshi T., Tian Y., Xu R., Heinemann S.H., and Hou S.. 2013a Mechanism of the modulation of BK potassium channel complexes with different auxiliary subunit compositions by the omega-3 fatty acid DHA. Proc. Natl. Acad. Sci. USA. 110:4822–4827. 10.1073/pnas.1222003110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Wissuwa B., Tian Y., Tajima N., Xu R., Bauer M., Heinemann S.H., and Hou S.. 2013b Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca2+-dependent K+ channels. Proc. Natl. Acad. Sci. USA. 110:4816–4821. 10.1073/pnas.1221997110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S., Heinemann S.H., and Hoshi T.. 2009. Modulation of BKCa channel gating by endogenous signaling molecules. Physiology (Bethesda). 24:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houamed K.M., Sweet I.R., and Satin L.S.. 2010. BK channels mediate a novel ionic mechanism that regulates glucose-dependent electrical activity and insulin secretion in mouse pancreatic β-cells. J. Physiol. 588:3511–3523. 10.1113/jphysiol.2009.184341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.W., Huang C.C., and Wu S.N.. 2007. Activation by zonisamide, a newer antiepileptic drug, of large-conductance calcium-activated potassium channel in differentiated hippocampal neuron-derived H19-7 cells. J. Pharmacol. Exp. Ther. 321:98–106. 10.1124/jpet.106.116954 [DOI] [PubMed] [Google Scholar]
- Imlach W.L., Finch S.C., Dunlop J., Meredith A.L., Aldrich R.W., and Dalziel J.E.. 2008. The molecular mechanism of “ryegrass staggers,” a neurological disorder of K+ channels. J. Pharmacol. Exp. Ther. 327:657–664. 10.1124/jpet.108.143933 [DOI] [PubMed] [Google Scholar]
- Imlach W.L., Finch S.C., Miller J.H., Meredith A.L., and Dalziel J.E. (2010). A role for BK channels in heart rate regulation in rodents. PLoS One 5, e8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe D.B., Wang B., and Brenner R.. 2011. Shaping of action potentials by type I and type II large-conductance Ca2+-activated K+ channels. Neuroscience. 192:205–218. 10.1016/j.neuroscience.2011.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar J.H., Porter V.A., Lederer W.J., and Nelson M.T.. 2000. Calcium sparks in smooth muscle. Am. J. Physiol. Cell Physiol. 278:C235–C256. 10.1152/ajpcell.2000.278.2.C235 [DOI] [PubMed] [Google Scholar]
- Jin W., Sugaya A., Tsuda T., Ohguchi H., and Sugaya E.. 2000. Relationship between large conductance calcium-activated potassium channel and bursting activity. Brain Res. 860:21–28. 10.1016/S0006-8993(00)01943-0 [DOI] [PubMed] [Google Scholar]
- Kaczorowski G.J., and Garcia M.L.. 2016. Developing Molecular Pharmacology of BK Channels for Therapeutic Benefit. Int. Rev. Neurobiol. 128:439–475. 10.1016/bs.irn.2016.02.013 [DOI] [PubMed] [Google Scholar]
- Kent J., and Meredith A.L.. 2008. BK channels regulate spontaneous action potential rhythmicity in the suprachiasmatic nucleus. PLoS One. 3:e3884 10.1371/journal.pone.0003884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.B. 2014. Channelopathies. Korean J. Pediatr. 57:1–18. 10.3345/kjp.2014.57.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus H.G., Schwarzer C., Koch R.O., Eberhart A., Kaczorowski G.J., Glossmann H., Wunder F., Pongs O., Garcia M.L., and Sperk G.. 1996. Distribution of high-conductance Ca2+-activated K+ channels in rat brain: targeting to axons and nerve terminals. J. Neurosci. 16:955–963. 10.1523/JNEUROSCI.16-03-00955.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong S.W., Lee I.H., Liu X., Hirschhorn J.N., and Mandl K.D.. 2018. Measuring coverage and accuracy of whole-exome sequencing in clinical context. Genet. Med. 20:1617–1626. 10.1038/gim.2018.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle B.D., and Braun A.P.. 2014. The regulation of BK channel activity by pre- and post-translational modifications. Front. Physiol. 5:316 10.3389/fphys.2014.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.H., Wu Y., Gao Z., Anderson M.E., Dalziel J.E., and Meredith A.L.. 2014. BK channels regulate sinoatrial node firing rate and cardiac pacing in vivo. Am. J. Physiol. Heart Circ. Physiol. 307:H1327–H1338. 10.1152/ajpheart.00354.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmikanthcharan S., Hisham M., Chaitanya Juluri S.K., and Nandakumar S.M.. 2018. Verapamil as an Adjuvant Treatment for Drug-Resistant Epilepsy. Indian J. Crit. Care Med. 22:680–682. 10.4103/ijccm.IJCCM_250_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., and Miller C.. 1983. Conduction and selectivity in potassium channels. J. Membr. Biol. 71:11–30. 10.1007/BF01870671 [DOI] [PubMed] [Google Scholar]
- Latorre R., Castillo K., Carrasquel-Ursulaez W., Sepulveda R.V., Gonzalez-Nilo F., Gonzalez C., and Alvarez O.. 2017. Molecular Determinants of BK Channel Functional Diversity and Functioning. Physiol. Rev. 97:39–87. 10.1152/physrev.00001.2016 [DOI] [PubMed] [Google Scholar]
- Laumonnier F., Roger S., Guérin P., Molinari F., M’rad R., Cahard D., Belhadj A., Halayem M., Persico A.M., Elia M., et al. . 2006. Association of a functional deficit of the BKCa channel, a synaptic regulator of neuronal excitability, with autism and mental retardation. Am. J. Psychiatry. 163:1622–1629. 10.1176/ajp.2006.163.9.1622 [DOI] [PubMed] [Google Scholar]
- Lee U.S., and Cui J.. 2010. BK channel activation: structural and functional insights. Trends Neurosci. 33:415–423. 10.1016/j.tins.2010.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., et al. Exome Aggregation Consortium . 2016. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 536:285–291. 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Poschmann S., Chen Q., Fazeli W., Oundjian N.J., Snoeijen-Schouwenaars F.M., Fricke O., Kamsteeg E.J., Willemsen M., and Wang Q.K.. 2018. De novo BK channel variant causes epilepsy by affecting voltage gating but not Ca2+ sensitivity. Eur. J. Hum. Genet. 26:220–229. 10.1038/s41431-017-0073-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Lorca R.A., Ma X., Rhodes A., and England S.K.. 2014. BK channels regulate myometrial contraction by modulating nuclear translocation of NF-κB. Endocrinology. 155:3112–3122. 10.1210/en.2014-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L., Li X., Moutton S., Schrier Vergano S.A., Cogne B., De Saint-Martin A., Hurst A.C.E., Hu Y., Bodamer O., Thevenon J., et al. . 2019. De novo loss-of-function KCNMA1 variants are associated with a new multiple malformation syndrome and a broad spectrum of developmental and neurological phenotypes. Hum. Mol. Genet. 10.1093/hmg/ddz117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Ye J., Zou X., Xu Z., Feng Y., Zou X., Chen Z., Li Y., and Cang Y.. 2014. CRL4A(CRBN) E3 ubiquitin ligase restricts BK channel activity and prevents epileptogenesis. Nat. Commun. 5:3924 10.1038/ncomms4924 [DOI] [PubMed] [Google Scholar]
- Liu R., Zhang Z., Liu H., Hou P., Lang J., Wang S., Yan H., Li P., Huang Z., Wu H., et al. . 2013. Human β-defensin 2 is a novel opener of Ca2+-activated potassium channels and induces vasodilation and hypotension in monkeys. Hypertension. 62:415–425. 10.1161/HYPERTENSIONAHA.111.01076 [DOI] [PubMed] [Google Scholar]
- Liu W., Morimoto T., Woda C., Kleyman T.R., and Satlin L.M.. 2007. Ca2+ dependence of flow-stimulated K secretion in the mammalian cortical collecting duct. Am. J. Physiol. Renal Physiol. 293:F227–F235. 10.1152/ajprenal.00057.2007 [DOI] [PubMed] [Google Scholar]
- Ly C., Melman T., Barth A.L., and Ermentrout G.B.. 2011. Phase-resetting curve determines how BK currents affect neuronal firing. J. Comput. Neurosci. 30:211–223. 10.1007/s10827-010-0246-3 [DOI] [PubMed] [Google Scholar]
- Maison S.F., Pyott S.J., Meredith A.L., and Liberman M.C.. 2013. Olivocochlear suppression of outer hair cells in vivo: evidence for combined action of BK and SK2 channels throughout the cochlea. J. Neurophysiol. 109:1525–1534. 10.1152/jn.00924.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares D., Srinivasan M., Salathe S.T., Ivonnet P., Baumlin N., Dennis J.S., Conner G.E., and Salathe M.. 2014. IFN-γ-mediated reduction of large-conductance, Ca2+-activated, voltage-dependent K+ (BK) channel activity in airway epithelial cells leads to mucociliary dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 306:L453–L462. 10.1152/ajplung.00247.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G., Puig S., Pietrzykowski A., Zadek P., Emery P., and Treistman S.. 2004. Somatic localization of a specific large-conductance calcium-activated potassium channel subtype controls compartmentalized ethanol sensitivity in the nucleus accumbens. J. Neurosci. 24:6563–6572. 10.1523/JNEUROSCI.0684-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y., Gallacher D.V., and Petersen O.H.. 1983. Voltage and Ca2+-activated K+ channel in baso-lateral acinar cell membranes of mammalian salivary glands. Nature. 302:827–829. 10.1038/302827a0 [DOI] [PubMed] [Google Scholar]
- McCobb D.P., Fowler N.L., Featherstone T., Lingle C.J., Saito M., Krause J.E., and Salkoff L.. 1995. A human calcium-activated potassium channel gene expressed in vascular smooth muscle. Am. J. Physiol. 269:H767–H777. [DOI] [PubMed] [Google Scholar]
- McManus O.B., and Rothberg B.S.. 2014. An old probe sheds new light on BK channel pore structure. J. Gen. Physiol. 144:499–501. 10.1085/jgp.201411306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O.B., Harris G.H., Giangiacomo K.M., Feigenbaum P., Reuben J.P., Addy M.E., Burka J.F., Kaczorowski G.J., and Garcia M.L.. 1993. An activator of calcium-dependent potassium channels isolated from a medicinal herb. Biochemistry. 32:6128–6133. 10.1021/bi00075a002 [DOI] [PubMed] [Google Scholar]
- Meera P., Wallner M., Song M., and Toro L.. 1997. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0-S6), an extracellular N terminus, and an intracellular (S9-S10) C terminus. Proc. Natl. Acad. Sci. USA. 94:14066–14071. 10.1073/pnas.94.25.14066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehranfard N., Gholamipour-Badie H., Motamedi F., Janahmadi M., and Naderi N.. 2014. The effect of paxilline on early alterations of electrophysiological properties of dentate gyrus granule cells in pilocarpine-treated rats. Iran. J. Pharm. Res. 13(Suppl):125–132. [PMC free article] [PubMed] [Google Scholar]
- Mehranfard N., Gholamipour-Badie H., Motamedi F., Janahmadi M., and Naderi N.. 2015. Long-term increases in BK potassium channel underlie increased action potential firing in dentate granule neurons following pilocarpine-induced status epilepticus in rats. Neurosci. Lett. 585:88–91. 10.1016/j.neulet.2014.11.041 [DOI] [PubMed] [Google Scholar]
- Melman A., Bar-Chama N., McCullough A., Davies K., and Christ G.. 2007. Plasmid-based gene transfer for treatment of erectile dysfunction and overactive bladder: results of a phase I trial. Isr. Med. Assoc. J. 9:143–146. [PubMed] [Google Scholar]
- Meredith A.L. 2015. Genetic Methods for Studying Ion Channel Function in Physiology and Disease. In Handbook of Ion Channels. Trudeau M.C., and Zheng J., editors. CRC Press; 545–556. [Google Scholar]
- Meredith A.L., Thorneloe K.S., Werner M.E., Nelson M.T., and Aldrich R.W.. 2004. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J. Biol. Chem. 279:36746–36752. 10.1074/jbc.M405621200 [DOI] [PubMed] [Google Scholar]
- Meredith A.L., Wiler S.W., Miller B.H., Takahashi J.S., Fodor A.A., Ruby N.F., and Aldrich R.W.. 2006. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat. Neurosci. 9:1041–1049. 10.1038/nn1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H., Menegola M., Buchwalder L., Park E.W., Meredith A., Rhodes K.J., Aldrich R.W., and Trimmer J.S.. 2006. Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J. Comp. Neurol. 496:289–302. 10.1002/cne.20931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J.R., and Meredith A.L.. 2012. Genetic activation of BK currents in vivo generates bidirectional effects on neuronal excitability. Proc. Natl. Acad. Sci. USA. 109:18997–19002. 10.1073/pnas.1205573109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J.R., Whitt J.P., Wright B.N., Lai M.H., and Meredith A.L.. 2013. Mis-expression of the BK K+ channel disrupts suprachiasmatic nucleus circuit rhythmicity and alters clock-controlled behavior. Am. J. Physiol. Cell Physiol. 304:C299–C311. 10.1152/ajpcell.00302.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J.P., Zakharov S.I., Liu G., Yang L., Sok A.J., and Marx S.O.. 2006. Defining the BK channel domains required for beta1-subunit modulation. Proc. Natl. Acad. Sci. USA. 103:5096–5101. 10.1073/pnas.0600907103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Gouemo P., Faingold C.L., and Morad M.. 2009. Calcium channel dysfunction in inferior colliculus neurons of the genetically epilepsy-prone rat. Neuropharmacology. 56:665–675. 10.1016/j.neuropharm.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj C., Tang B., Nagy B.M., Papp R., Jain P.P., Marsh L.M., Meredith A.L., Ghanim B., Klepetko W., Kwapiszewska G., et al. . 2016. Docosahexaenoic acid causes rapid pulmonary arterial relaxation via KCa channel-mediated hyperpolarisation in pulmonary hypertension. Eur. Respir. J. 48:1127–1136. 10.1183/13993003.01814-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan J., Frech R., Walters S., Patel V., Frigerio R., and Maraganore D.M.. 2016. Low dose verapamil as an adjunct therapy for medically refractory epilepsy - An open label pilot study. Epilepsy Res. 126:197–200. 10.1016/j.eplepsyres.2016.07.004 [DOI] [PubMed] [Google Scholar]
- Nardi A., Calderone V., Chericoni S., and Morelli I.. 2003. Natural modulators of large-conductance calcium-activated potassium channels. Planta Med. 69:885–892. 10.1055/s-2003-45095 [DOI] [PubMed] [Google Scholar]
- Nelson A.B., Faulstich M., Moghadam S., Onori K., Meredith A., and du Lac S.. 2017. BK Channels Are Required for Multisensory Plasticity in the Oculomotor System. Neuron. 93:211–220. 10.1016/j.neuron.2016.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J., and Miller C.. 1988. Discrete Ba2+ block as a probe of ion occupancy and pore structure in the high-conductance Ca2+ -activated K+ channel. J. Gen. Physiol. 92:569–586. 10.1085/jgp.92.5.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen S.P., Munch E., Moldt P., and Drejer J.. 1994a Selective activation of Ca2+-dependent K+ channels by novel benzimidazolone. Eur. J. Pharmacol. 251:53–59. 10.1016/0014-2999(94)90442-1 [DOI] [PubMed] [Google Scholar]
- Olesen S.P., Munch E., Wätjen F., and Drejer J.. 1994b NS 004-an activator of Ca2+-dependent K+ channels in cerebellar granule cells. Neuroreport. 5:1001–1004. 10.1097/00001756-199404000-00037 [DOI] [PubMed] [Google Scholar]
- Ovsiew F., Meador K.J., and Sethi K.. 1998. Verapamil for severe hyperkinetic movement disorders. Mov. Disord. 13:341–344. 10.1002/mds.870130224 [DOI] [PubMed] [Google Scholar]
- Pacheco Otalora L.F., Hernandez E.F., Arshadmansab M.F., Francisco S., Willis M., Ermolinsky B., Zarei M., Knaus H.G., and Garrido-Sanabria E.R.. 2008. Down-regulation of BK channel expression in the pilocarpine model of temporal lobe epilepsy. Brain Res. 1200:116–131. 10.1016/j.brainres.2008.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanck L., and Ganetzky B.. 1994. Cloning and characterization of human and mouse homologs of the Drosophila calcium-activated potassium channel gene, slowpoke Hum. Mol. Genet. 3:1239–1243. 10.1093/hmg/3.8.1239 [DOI] [PubMed] [Google Scholar]
- Pallotta B.S., Magleby K.L., and Barrett J.N.. 1981. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 293:471–474. 10.1038/293471a0 [DOI] [PubMed] [Google Scholar]
- Petrik D., Wang B., and Brenner R.. 2011. Modulation by the BK accessory β4 subunit of phosphorylation-dependent changes in excitability of dentate gyrus granule neurons. Eur. J. Neurosci. 34:695–704. 10.1111/j.1460-9568.2011.07799.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzykowski A.Z., Friesen R.M., Martin G.E., Puig S.I., Nowak C.L., Wynne P.M., Siegelmann H.T., and Treistman S.N.. 2008. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 59:274–287. 10.1016/j.neuron.2008.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante A.E., Moldenhauer H.J., Harvey J.R.M., and Meredith A.L.. 2019. Gain-of-Function Effects of KCNMA1-N999S Mutation on Human BK Channel Properties, in: 63rd Annual Meeting of the Biophysical Society. [Google Scholar]
- Pourmasoumi M., Vosoughi N., Derakhshandeh-Rishehri S.M., Assarroudi M., and Heidari-Beni M.. 2018. Association of Omega-3 Fatty Acid and Epileptic Seizure in Epileptic Patients: A Systematic Review. Int. J. Prev. Med. 9:36 10.4103/ijpvm.IJPVM_281_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulst S.M. 1999. Genetic linkage analysis. Arch. Neurol. 56:667–672. 10.1001/archneur.56.6.667 [DOI] [PubMed] [Google Scholar]
- Pyott S.J., and Duncan R.K.. 2016. BK Channels in the Vertebrate Inner Ear. Int. Rev. Neurobiol. 128:369–399. 10.1016/bs.irn.2016.03.016 [DOI] [PubMed] [Google Scholar]
- Pyott S.J., Meredith A.L., Fodor A.A., Vázquez A.E., Yamoah E.N., and Aldrich R.W.. 2007. Cochlear function in mice lacking the BK channel alpha, beta1, or beta4 subunits. J. Biol. Chem. 282:3312–3324. 10.1074/jbc.M608726200 [DOI] [PubMed] [Google Scholar]
- Raffaelli G., Saviane C., Mohajerani M.H., Pedarzani P., and Cherubini E.. 2004. BK potassium channels control transmitter release at CA3-CA3 synapses in the rat hippocampus. J. Physiol. 557:147–157. 10.1113/jphysiol.2004.062661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieg T., Vallon V., Sausbier M., Sausbier U., Kaissling B., Ruth P., and Osswald H.. 2007. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int. 72:566–573. 10.1038/sj.ki.5002369 [DOI] [PubMed] [Google Scholar]
- Robitaille R., Adler E.M., and Charlton M.P.. 1993. Calcium channels and calcium-gated potassium channels at the frog neuromuscular junction. J. Physiol. Paris. 87:15–24. 10.1016/0928-4257(93)90020-T [DOI] [PubMed] [Google Scholar]
- Rohmann K.N., Wersinger E., Braude J.P., Pyott S.J., and Fuchs P.A.. 2015. Activation of BK and SK channels by efferent synapses on outer hair cells in high-frequency regions of the rodent cochlea. J. Neurosci. 35:1821–1830. 10.1523/JNEUROSCI.2790-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Morayo Akande A., Large R.J., Webb T.I., Camarasu C., Sergeant G.P., McHale N.G., Thornbury K.D., and Hollywood M.A.. 2012. Structure-activity relationships of a novel group of large-conductance Ca2+-activated K+ (BK) channel modulators: the GoSlo-SR family. ChemMedChem. 7:1763–1769. 10.1002/cmdc.201200321 [DOI] [PubMed] [Google Scholar]
- Sausbier M., Hu H., Arntz C., Feil S., Kamm S., Adelsberger H., Sausbier U., Sailer C.A., Feil R., Hofmann F., et al. . 2004. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc. Natl. Acad. Sci. USA. 101:9474–9478. 10.1073/pnas.0401702101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausbier M., Arntz C., Bucurenciu I., Zhao H., Zhou X.B., Sausbier U., Feil S., Kamm S., Essin K., Sailer C.A., et al. . 2005. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation. 112:60–68. 10.1161/01.CIR.0000156448.74296.FE [DOI] [PubMed] [Google Scholar]
- Sausbier M., Matos J.E., Sausbier U., Beranek G., Arntz C., Neuhuber W., Ruth P., and Leipziger J.. 2006a Distal colonic K+ secretion occurs via BK channels. J. Am. Soc. Nephrol. 17:1275–1282. 10.1681/ASN.2005101111 [DOI] [PubMed] [Google Scholar]
- Sausbier M., Zhou X.B., Beier C., Sausbier U., Wolpers D., Maget S., Martin C., Dietrich A., Ressmeyer A.R., Renz H., et al. . 2007. Reduced rather than enhanced cholinergic airway constriction in mice with ablation of the large conductance Ca2+-activated K+ channel. FASEB J. 21:812–822. 10.1096/fj.06-7167com [DOI] [PubMed] [Google Scholar]
- Sausbier U., Sausbier M., Sailer C.A., Arntz C., Knaus H.G., Neuhuber W., and Ruth P.. 2006b Ca2+-activated K+ channels of the BK-type in the mouse brain. Histochem. Cell Biol. 125:725–741. 10.1007/s00418-005-0124-7 [DOI] [PubMed] [Google Scholar]
- Sausbier U., Dullin C., Missbach-Guentner J., Kabagema C., Flockerzie K., Kuscher G.M., Stuehmer W., Neuhuber W., Ruth P., Alves F., and Sausbier M.. 2011. Osteopenia due to enhanced cathepsin K release by BK channel ablation in osteoclasts. PLoS One. 6:e21168 10.1371/journal.pone.0021168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savina T.A., Levin S.G., Poletaeva I.I., Fedotova I.B., and Shchipakina T.G.. 2014. Audiogenic kindling changes the subunit composition of BK-channels in dentate gyrus of Krushinskii-Molodkina rats. Biochemistry (Moscow). Supplement Series A: Membrane and Cell Biology. 8:111–115. [Google Scholar]
- Schuckit M.A., Wilhelmsen K., Smith T.L., Feiler H.S., Lind P., Lange L.A., and Kalmijn J.. 2005. Autosomal linkage analysis for the level of response to alcohol. Alcohol. Clin. Exp. Res. 29:1976–1982. 10.1097/01.alc.0000187598.82921.27 [DOI] [PubMed] [Google Scholar]
- Shao L.R., Halvorsrud R., Borg-Graham L., and Storm J.F.. 1999. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J. Physiol. 521:135–146. 10.1111/j.1469-7793.1999.00135.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan J.J., Benedetti B.L., and Barth A.L.. 2009. Anticonvulsant effects of the BK-channel antagonist paxilline. Epilepsia. 50:711–720. 10.1111/j.1528-1167.2008.01888.x [DOI] [PubMed] [Google Scholar]
- Shruti S., Clem R.L., and Barth A.L.. 2008. A seizure-induced gain-of-function in BK channels is associated with elevated firing activity in neocortical pyramidal neurons. Neurobiol. Dis. 30:323–330. 10.1016/j.nbd.2008.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira-Moriyama L., Kovac S., Kurian M.A., Houlden H., Lees A.J., Walker M.C., Roze E., Paciorkowski A.R., Mink J.W., and Warner T.T.. 2018. Phenotypes, genotypes, and the management of paroxysmal movement disorders. Dev. Med. Child Neurol. 60:559–565. 10.1111/dmcn.13744 [DOI] [PubMed] [Google Scholar]
- Soder R.P., and Petkov G.V.. 2011. Large conductance Ca2+-activated K+ channel activation with NS1619 decreases myogenic and neurogenic contractions of rat detrusor smooth muscle. Eur. J. Pharmacol. 670:252–259. 10.1016/j.ejphar.2011.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro C.R., Prakriya M., Ding J.P., and Lingle C.J.. 1995. Inactivating and noninactivating Ca2+- and voltage-dependent K+ current in rat adrenal chromaffin cells. J. Neurosci. 15:6110–6123. 10.1523/JNEUROSCI.15-09-06110.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen M.V., Matos J.E., Sausbier M., Sausbier U., Ruth P., Praetorius H.A., and Leipziger J.. 2008. Aldosterone increases KCa1.1 (BK) channel-mediated colonic K+ secretion. J. Physiol. 586:4251–4264. 10.1113/jphysiol.2008.156968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spektor M., Rodriguez R., Rosenbaum R.S., Wang H.Z., Melman A., and Christ G.J.. 2002. Potassium channels and human corporeal smooth muscle cell tone: further evidence of the physiological relevance of the Maxi-K channel subtype to the regulation of human corporeal smooth muscle tone in vitro. J. Urol. 167:2628–2635. 10.1016/S0022-5347(05)65049-5 [DOI] [PubMed] [Google Scholar]
- Sprossmann F., Pankert P., Sausbier U., Wirth A., Zhou X.B., Madlung J., Zhao H., Bucurenciu I., Jakob A., Lamkemeyer T., et al. . 2009. Inducible knockout mutagenesis reveals compensatory mechanisms elicited by constitutive BK channel deficiency in overactive murine bladder. FEBS J. 276:1680–1697. 10.1111/j.1742-4658.2009.06900.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staisch J., Du X., Carvalho-De-Souza J., Kubota T., Bezanilla F., and Gomez C.. 2016. A Mutation Causing Reduced BK Channel Activity Leads to Cognitive Impairment and Progressive Cerebellar Ataxia. Neurology. 86:P5.394. [Google Scholar]
- Stefani E., Ottolia M., Noceti F., Olcese R., Wallner M., Latorre R., and Toro L.. 1997. Voltage-controlled gating in a large conductance Ca2+-sensitive K+channel (hslo). Proc. Natl. Acad. Sci. USA. 94:5427–5431. 10.1073/pnas.94.10.5427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinlein O.K. 2008. Genetics and epilepsy. Dialogues Clin. Neurosci. 10:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm J.F. 1987. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J. Physiol. 385:733–759. 10.1113/jphysiol.1987.sp016517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stummann T.C., Poulsen J.H., Hay-Schmidt A., Grunnet M., Klaerke D.A., Rasmussen H.B., Olesen S.P., and Jorgensen N.K.. 2003. Pharmacological investigation of the role of ion channels in salivary secretion. Pflugers Arch. 446:78–87. 10.1007/s00424-002-0985-8 [DOI] [PubMed] [Google Scholar]
- Tabarki B., AlMajhad N., AlHashem A., Shaheen R., and Alkuraya F.S.. 2016. Homozygous KCNMA1 mutation as a cause of cerebellar atrophy, developmental delay and seizures. Hum. Genet. 135:1295–1298. 10.1007/s00439-016-1726-y [DOI] [PubMed] [Google Scholar]
- Tang X.D., Xu R., Reynolds M.F., Garcia M.L., Heinemann S.H., and Hoshi T.. 2003. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature. 425:531–535. 10.1038/nature02003 [DOI] [PubMed] [Google Scholar]
- Tanimoto N., Sothilingam V., Euler T., Ruth P., Seeliger M.W., and Schubert T.. 2012. BK channels mediate pathway-specific modulation of visual signals in the in vivo mouse retina. J. Neurosci. 32:4861–4866. 10.1523/JNEUROSCI.4654-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M.R., and Beeton C.. 2018. Differences in ion channel phenotype and function between humans and animal models. Front. Biosci. 23:43–64. 10.2741/4581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme H., Stumpff F., Ottlecz A., Percicot C.L., Lambrou G.N., and Wiederholt M.. 2001. Mechanisms of action of unoprostone on trabecular meshwork contractility. Invest. Ophthalmol. Vis. Sci. 42:3193–3201. [PubMed] [Google Scholar]
- Thorneloe K.S., Meredith A.L., Knorn A.M., Aldrich R.W., and Nelson M.T.. 2005. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am. J. Physiol. Renal Physiol. 289:F604–F610. 10.1152/ajprenal.00060.2005 [DOI] [PubMed] [Google Scholar]
- Tomás M., Vázquez E., Fernández-Fernández J.M., Subirana I., Plata C., Heras M., Vila J., Marrugat J., Valverde M.A., and Sentí M.. 2008. Genetic variation in the KCNMA1 potassium channel alpha subunit as risk factor for severe essential hypertension and myocardial infarction. J. Hypertens. 26:2147–2153. 10.1097/HJH.0b013e32831103d8 [DOI] [PubMed] [Google Scholar]
- Trépanier M.O., Lim J., Lai T.K., Cho H.J., Domenichiello A.F., Chen C.T., Taha A.Y., Bazinet R.P., and Burnham W.M.. 2014. Intraperitoneal administration of docosahexaenoic acid for 14 days increases serum unesterified DHA and seizure latency in the maximal pentylenetetrazol model. Epilepsy Behav. 33:138–143. 10.1016/j.yebeh.2014.02.020 [DOI] [PubMed] [Google Scholar]
- Tricarico D., and Mele A.. 2017. Commentary: A BK (Slo1) channel journey from molecule to physiology. Front. Pharmacol. 8:188 10.3389/fphar.2017.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricarico D., Barbieri M., Mele A., Carbonara G., and Camerino D.C.. 2004. Carbonic anhydrase inhibitors are specific openers of skeletal muscle BK channel of K+-deficient rats. FASEB J. 18:760–761. 10.1096/fj.03-0722fje [DOI] [PubMed] [Google Scholar]
- Typlt M., Mirkowski M., Azzopardi E., Ruettiger L., Ruth P., and Schmid S.. 2013a Mice with deficient BK channel function show impaired prepulse inhibition and spatial learning, but normal working and spatial reference memory. PLoS One. 8:e81270 10.1371/journal.pone.0081270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Typlt M., Mirkowski M., Azzopardi E., Ruth P., Pilz P.K., and Schmid S.. 2013b Habituation of reflexive and motivated behavior in mice with deficient BK channel function. Front. Integr. Nuerosci. 7:79 10.3389/fnint.2013.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A., et al. . 2015. Proteomics. Tissue-based map of the human proteome. Science. 347:1260419 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- Unterberger I., and Trinka E.. 2008. Diagnosis and treatment of paroxysmal dyskinesias revisited. Ther. Adv. Neurol. Disorder. 1:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde M.A., Rojas P., Amigo J., Cosmelli D., Orio P., Bahamonde M.I., Mann G.E., Vergara C., and Latorre R.. 1999. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 285:1929–1931. 10.1126/science.285.5435.1929 [DOI] [PubMed] [Google Scholar]
- Vang A., Mazer J., Casserly B., and Choudhary G.. 2010. Activation of endothelial BKCa channels causes pulmonary vasodilation. Vascul. Pharmacol. 53:122–129. 10.1016/j.vph.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Van Goor F., Li Y.X., and Stojilkovic S.S.. 2001. Paradoxical role of large-conductance calcium-activated K+ (BK) channels in controlling action potential-driven Ca2+ entry in anterior pituitary cells. J. Neurosci. 21:5902–5915. 10.1523/JNEUROSCI.21-16-05902.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M., Meera P., and Toro L.. 1996. Determinant for beta-subunit regulation in high-conductance voltage-activated and Ca2+-sensitive K+ channels: an additional transmembrane region at the N terminus. Proc. Natl. Acad. Sci. USA. 93:14922–14927. 10.1073/pnas.93.25.14922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., and Sigworth F.J.. 2009. Structure of the BK potassium channel in a lipid membrane from electron cryomicroscopy. Nature. 461:292–295. 10.1038/nature08291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Rothberg B.S., and Brenner R.. 2009. Mechanism of increased BK channel activation from a channel mutation that causes epilepsy. J. Gen. Physiol. 133:283–294. 10.1085/jgp.200810141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Jaffe D.B., and Brenner R.. 2014. Current understanding of iberiotoxin-resistant BK channels in the nervous system. Front. Physiol. 5:382 10.3389/fphys.2014.00382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Bugay V., Ling L., Chuang H.H., Jaffe D.B., and Brenner R.. 2016. Knockout of the BK β4-subunit promotes a functional coupling of BK channels and ryanodine receptors that mediate a fAHP-induced increase in excitability. J. Neurophysiol. 116:456–465. 10.1152/jn.00857.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yu S., Zhang Q., Chen Y., Bao X., and Wu X.. 2017. KCNMA1 mutation in children with paroxysmal dyskinesia and epilepsy: Case report and literature review. Transl. Sci. Rare Dis. 2:8. [Google Scholar]
- Wang Y.J., Lin M.W., Lin A.A., and Wu S.N.. 2008. Riluzole-induced block of voltage-gated Na+ current and activation of BKCa channels in cultured differentiated human skeletal muscle cells. Life Sci. 82:11–20. 10.1016/j.lfs.2007.10.015 [DOI] [PubMed] [Google Scholar]
- Wanner S.G., Koch R.O., Koschak A., Trieb M., Garcia M.L., Kaczorowski G.J., and Knaus H.G.. 1999. High-conductance calcium-activated potassium channels in rat brain: pharmacology, distribution, and subunit composition. Biochemistry. 38:5392–5400. 10.1021/bi983040c [DOI] [PubMed] [Google Scholar]
- Werner M.E., Zvara P., Meredith A.L., Aldrich R.W., and Nelson M.T.. 2005. Erectile dysfunction in mice lacking the large-conductance calcium-activated potassium (BK) channel. J. Physiol. 567:545–556. 10.1113/jphysiol.2005.093823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M.E., Meredith A.L., Aldrich R.W., and Nelson M.T.. 2008. Hypercontractility and impaired sildenafil relaxations in the BKCa channel deletion model of erectile dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295:R181–R188. 10.1152/ajpregu.00173.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R.S., Zemen B.G., Khan Z., Montgomery J.R., Herrera G.M., and Meredith A.L.. 2015. Evaluation of mouse urinary bladder smooth muscle for diurnal differences in contractile properties. Front. Pharmacol. 5:293 10.3389/fphar.2014.00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmire L.E., Ling L., Bugay V., Carver C.M., Timilsina S., Chuang H.H., Jaffe D.B., Shapiro M.S., Cavazos J.E., and Brenner R.. 2017. Downregulation of KCNMB4 expression and changes in BK channel subtype in hippocampal granule neurons following seizure activity. PLoS One. 12:e0188064 10.1371/journal.pone.0188064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt J.P., Montgomery J.R., and Meredith A.L.. 2016. BK channel inactivation gates daytime excitability in the circadian clock. Nat. Commun. 7:10837 10.1038/ncomms10837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt J.P., McNally B.A., and Meredith A.L.. 2018. Differential contribution of Ca2+ sources to day and night BK current activation in the circadian clock. J. Gen. Physiol. 150:259–275. 10.1085/jgp.201711945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack M.D., Hoang C., and Khodakhah K.. 2009. Large conductance calcium-activated potassium channels affect both spontaneous firing and intracellular calcium concentration in cerebellar Purkinje neurons. Neuroscience. 162:989–1000. 10.1016/j.neuroscience.2009.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes R.C., and Lignani G.. 2018. Gene therapy and editing: Novel potential treatments for neuronal channelopathies. Neuropharmacology. 132:108–117. 10.1016/j.neuropharm.2017.05.029 [DOI] [PubMed] [Google Scholar]
- Xia X.M., Zeng X., and Lingle C.J.. 2002. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 418:880–884. 10.1038/nature00956 [DOI] [PubMed] [Google Scholar]
- Yan J., and Aldrich R.W.. 2010. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature. 466:513–516. 10.1038/nature09162 [DOI] [PubMed] [Google Scholar]
- Yan J., and Aldrich R.W.. 2012. BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc. Natl. Acad. Sci. USA. 109:7917–7922. 10.1073/pnas.1205435109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Zhang G., and Cui J.. 2015. BK channels: multiple sensors, one activation gate. Front. Physiol. 6:29 10.3389/fphys.2015.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Krishnamoorthy G., Saxena A., Zhang G., Shi J., Yang H., Delaloye K., Sept D., and Cui J.. 2010. An epilepsy/dyskinesia-associated mutation enhances BK channel activation by potentiating Ca2+ sensing. Neuron. 66:871–883. 10.1016/j.neuron.2010.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Li P.Y., Cheng J., Mao L., Wen J., Tan X.Q., Liu Z.F., and Zeng X.R.. 2013. Function of BKCa channels is reduced in human vascular smooth muscle cells from Han Chinese patients with hypertension. Hypertension. 61:519–525. 10.1161/HYPERTENSIONAHA.111.00211 [DOI] [PubMed] [Google Scholar]
- Yeşil G., Aralaşmak A., Akyüz E., İçağasıoğlu D., Uygur Şahin T., and Bayram Y.. 2018. Expanding the Phenotype of Homozygous KCNMA1 Mutations; Dyskinesia, Epilepsy, Intellectual Disability, Cerebellar and Corticospinal Tract Atrophy. Balkan Med. J. 35:336–339. 10.4274/balkanmedj.2017.0986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P., Leonetti M.D., Pico A.R., Hsiung Y., and MacKinnon R.. 2010. Structure of the human BK channel Ca2+-activation apparatus at 3.0 A resolution. Science. 329:182–186. 10.1126/science.1190414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P., Leonetti M.D., Hsiung Y., and MacKinnon R.. 2011. Open structure of the Ca2+ gating ring in the high-conductance Ca2+-activated K+ channel. Nature. 481:94–97. 10.1038/nature10670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue F., Cheng Y., Breschi A., Vierstra J., Wu W., Ryba T., Sandstrom R., Ma Z., Davis C., Pope B.D., et al. Mouse ENCODE Consortium . 2014. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 515:355–364. 10.1038/nature13992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.L., and Gold M.S.. 2009. Dihydropyridine block of voltage-dependent K+ currents in rat dorsal root ganglion neurons. Neuroscience. 161:184–194. 10.1016/j.neuroscience.2009.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Huang S.Y., Yang J., Shi J., Yang X., Moller A., Zou X., and Cui J.. 2010. Ion sensing in the RCK1 domain of BK channels. Proc. Natl. Acad. Sci. USA. 107:18700–18705. 10.1073/pnas.1010124107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.B., Tian M.Q., Gao K., Jiang Y.W., and Wu Y.. 2015. De novo KCNMA1 mutations in children with early-onset paroxysmal dyskinesia and developmental delay. Mov. Disord. 30:1290–1292. 10.1002/mds.26216 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.