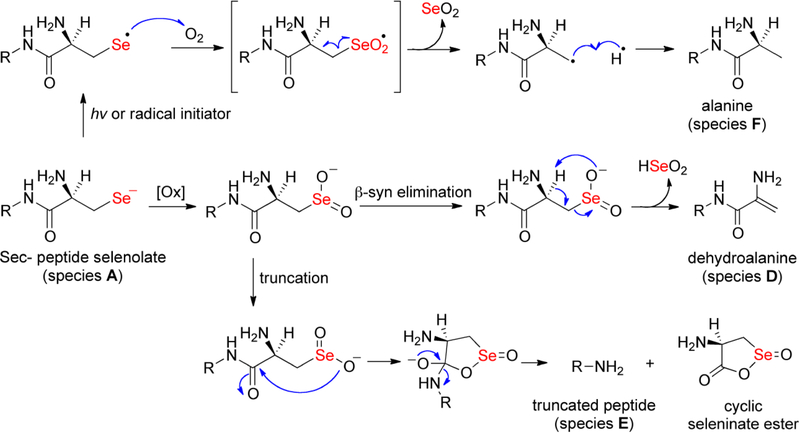
Figure 6: Potential chemical mechanisms of deselenization of peptide 12.
The selenol in the sample can undergo oxidation to form the seleninate, which then undergoes rapid β-syn elimination to produce the dehydroalanine-containing peptide (species D). The truncated peptide (species E) is potentially explained by attack of the nucleophilic seleninate oxygen onto the carbonyl of the amide backbone, resulting in a cyclic seleninate ester and elimination of the Sec residue (lower pathway). Species F can be produced through a radical mechanism. Initiation of the radical could be photo-induced or alternatively, the selenolate can react with molecular oxygen to produce the selanyl radical and superoxide. The selanyl radical could recombine with molecular oxygen resulting in a seleninyl radical that can break down into selenium dioxide (SeO2) and an alanine-containing peptide.