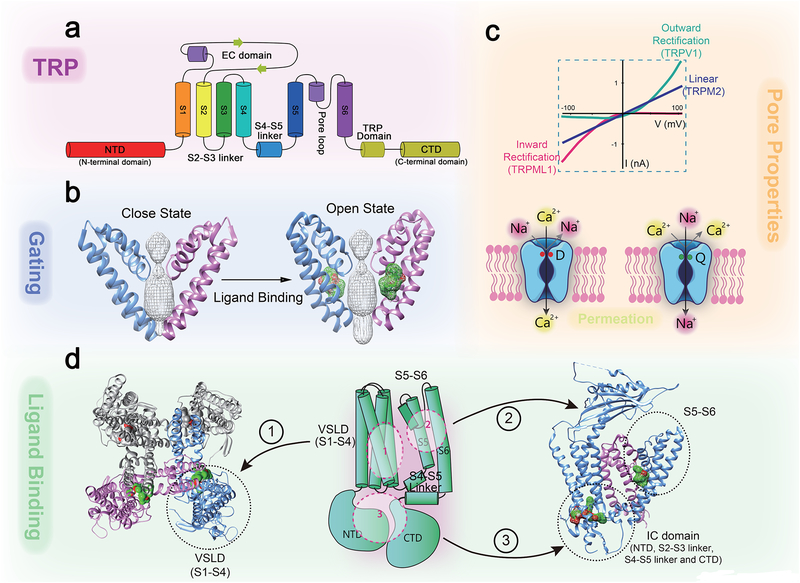
Fig. 1. Architecture and functional elements of a TRP channel.
Structural biology analyses reveal critical domains and amino acid residues for Ca2+ permeation and selectivity, and allow visualization of elements such as selectivity filter, activation gates, agonist binding sites, and explain gating-coupling machinery. a. TRPs are 6 transmembrane (TM, S1–S6) cation channels with N- and C- terminal domains facing the cytosol. b, Ligand binding to the S5–S6 domain leads to opening the lower S6 gate. c. Pore properties of TRP channels. Upper panel: representative TRP current-voltage (I-V) traces. Lower panels: the pore-loop between S5 and S6 forms the selectivity filter and upper gate of the channel. Negatively charged residues (e.g. Asp541 in TRPV6; red, the left panel) form the high-affinity Ca2+-binding sites required for Ca2+ permeation and selectivity. In TRPs with very low PCa, specific neutral and polar amino acid residues (e.g., Gln977 in TRPM4; green, the righ panel) form binding sites that favor monovalent cations over Ca2+. d. Ligand binding sites are localized in S1–4 VSLD (site 1), S5–S6 region (site 2; see agonist-bound cryo-EM structures of TRP in the zoomed-in image), or intracellular (IC) domains (site 3; see agonist-bound cryo-EM structures of TRP in the zoomed-in image). The S4–S5 linker and TRP domain act as the binding-gating coupling machinery that interacts to pull the S6 gate open upon ligand-binding.