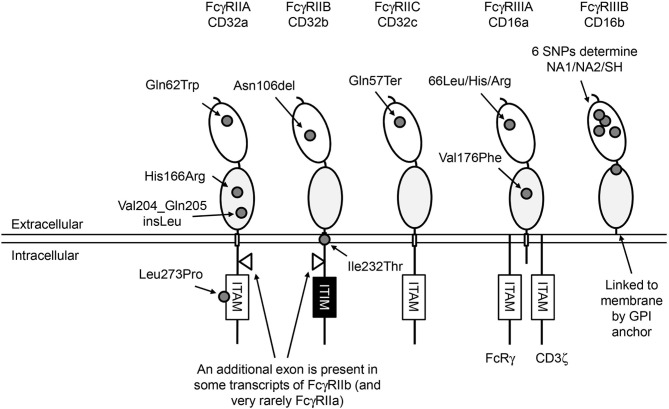
Figure 1.
Human FcγRs. Overview of the structure of human low-to-medium-affinity FcγRs. The oval shapes in the extracellular part of the FcγRs represent the different extracellular domains; the light gray domains are the domains where IgG molecules bind. All FcγRs except FcγRIIIB are linked to the plasma membrane by transmembrane (TM) domains indicated by small rectangles. FcγRIIIB is linked to the plasma membrane through a GPI anchor. FcγRIIIA has a small intracellular domain that associates with adaptor molecules that can initiate an intracellular signaling cascade when multiple FcγRs are cross-linked, which ultimately leads to activation of the cell on which the FcγRs are expressed. FcγRII receptors have a much larger intracellular domain, and contain a signaling motif to start this cascade in their own polypeptide chain. Signaling by activating FcγRs is mediated by immunoreceptor tyrosine-based activating motifs (ITAM) that are present either in the cytoplasmic tail of the receptor itself or in non-covalently associated signaling adaptor proteins, such as the common γ-chain (FcRγ). Aggregation of activating FcγR by binding of multivalent ligands, such as an opsonized pathogen or blood cell or an immune complex, results in the phosphorylation of ITAM tyrosine residues by Src family protein tyrosine kinases (PTKs), and ultimately leads to activation of cellular responses (1). Aggregation of the inhibitory FcγRIIB, which contains an immunoreceptor tyrosine-based inhibitory motif (ITIM), also results in phosphorylation of tyrosine residues by Src family PTKs. In contrast to ITAMs, phosphorylated ITIMs serve as binding sites for phosphotyrosine phosphatases (PTPs) which dephosphorylate other proteins resulting in inhibition of activating pathways (2). Approximate location of functional SNPs in the FcγRs are indicated by small gray circles, SNPs are indicated by 3-letter amino-acid codes. ITAM, immunoreceptor tyrosine-based activating motif; ITIM, immunoreceptor tyrosine-based inhibitory motif.