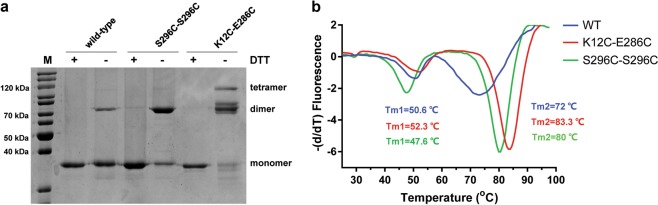
Fig. 2.
Cysteine mutations K12C–E286C and S296C introduced disulfide bonds between the AgUricase subunits and significantly improved the thermostability of AgUricase. a K12C–E286C and S296C–S296C AgUricase mutants formed disulfide bonds between subunits. Disulfide bridge formation was verified by SDS–PAGE with and without DTT. b Thermal shift assays (TSA) of AgUricases. The thermal stabilities of wild-type (blue), K12C–E286C (red), and S296C–C302S (green) mutant AgUricase were evaluated by TSA using the method described in the methods section