Fig. 7.
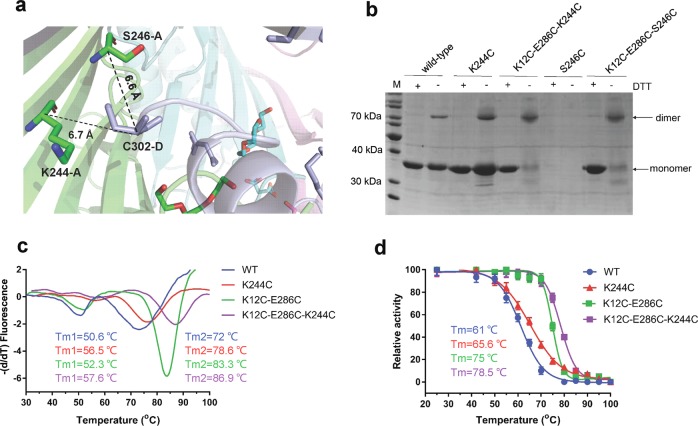
K244C forms disulfide bonds with C302 and improves the thermostability of AgUricase. a Cα–Cα distance between K244 and S246 in subunit A and C302 in subunit D. b SDS–PAGE results showing that mutation K244C strengthened dimer formation between the subunits in both the WT and K12C/E286C mutant AgUricases, whereas S246C did not. c TSA results showing that the K244C mutation shifted the Tm peaks of both the WT and K12C–E286C mutant AgUricases to higher temperatures. d The K244C mutation increased the thermostability of the WT and K12C–E286C mutant AgUricase