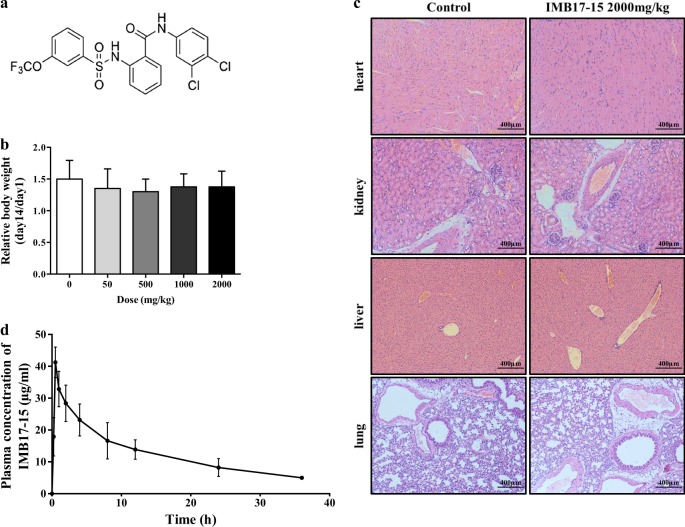
Fig. 1.
IMB17–15 showed no significant toxicity in KM mice. a The structure of IMB17–15. b KM mice were treated with different concentrations of IMB17–15 once by gavage and were continuously observed for 14 days. The relative body weight was calculated by the ratio of the final weight and the initial weight. This figure shows that IMB17–15 was not toxic in KM mice. c Histopathological examination of various organs (H&E staining, ×40) from KM mice with or without IMB17–15 treatment at a dose of 2000 mg/kg. No toxicopathological changes were found in the heart, liver, spleen, lung, and kidney. d Plasma concentration-time profiles of IMB17–15 after gavage administration at a single dose of 400 mg/kg in hamsters. KM mice Kunming mice. The values are expressed as the mean ± SD (n = 4 in each group for the acute oral toxicity study, n = 6 in each group for the pharmacokinetics in vivo study)