Abstract
Background/Aims
Interstitial cells play important roles in gastrointestinal (GI) neuro-smooth muscle transmission. The underlying mechanisms of colonic dysmotility have not been well illustrated. We established a partial colon obstruction (PCO) mouse model to investigate the changes of interstitial cells and the correlation with colonic motility.
Methods
Western blot technique was employed to observe the protein expressions of Kit, platelet-derived growth factor receptor-α (Pdgfra), Ca2+-activated Cl− (Ano1) channels, and small conductance Ca2+- activated K+ (SK) channels. Colonic migrating motor complexes (CMMCs) and isometric force measurements were employed in control mice and PCO mice.
Results
PCO mice showed distended abdomen and feces excretion was significantly reduced. Anatomically, the colon above the obstructive silicone ring was obviously dilated. Kit and Ano1 proteins in the colonic smooth muscle layer of the PCO colons were significantly decreased, while the expression of Pdgfra and SK3 proteins were significantly increased. The effects of a nitric oxide synthase inhibitor (L-NAME) and an Ano1 channel inhibitor (NPPB) on CMMC and colonic spontaneous contractions were decreased in the proximal and distal colons of PCO mice. The SK agonist, CyPPA and antagonist, apamin in PCO mice showed more effect to the CMMCs and colonic smooth muscle contractions.
Conclusions
Colonic transit disorder may be due to the downregulation of the Kit and Ano1 channels and the upregulation of SK3 channels in platelet-derived growth factor receptor-α positive (PDGFRα+) cells. The imbalance between interstitial cells of Cajal-Ano1 and PDGFRα-SK3 distribution might be a potential reason for the colonic dysmotility.
Keywords: Chloride channels, Interstitial cells of Cajal, Small-conductance calcium-activated potassium channels
Introduction
Gastrointestinal (GI) smooth muscles are composed of 3 types of cells, including smooth muscle cells, interstitial cells of Cajal (ICC), and platelet-derived growth factor receptor-α positive (PDGFRα+) cells, which is called the smooth muscle cells, ICC, and PDGFRα+ cells (SIP) syncytium.1–3
Myenteric ICCs (ICC-MY) have a pacemaker role to generate spontaneous electrical activity.4 Calcium activated chloride channels (Ano 1, a molecular candidate) are highly expressed in ICCs and may be a candidate conductance of pacemaker potentials.4,5 Intramuscular ICCs (ICC-IM) display close anatomical apposition to nerve varicosities.6 ICC-IM respond to excitatory and inhibitory neurotransmitters between neurons and smooth muscles.6 Acetylcholine, which is a major excitatory neurotransmitter induced depolarization by activation of Ano1 currents. The effect of nitric oxide (NO), an inhibitory neurotransmitter, is also mediated by ICC-IM, however the ionic conductance activated or inhibited by NO has not been clarified.
PDGFRα+ cells were identified in the muscle layers of the GI tract and showed close apposition with enteric motor neurons.7 These cells are mainly distributed in the muscle layers of murine and human colon and mediate purinergic inhibitory neurotransmission.7,8 PDGFRα+ cells uniquely express the purinergic P2Y1 receptor and small-conductance Ca2+-activated K+ channels (a molecular candidate Kcnn3, SK3) which are mainly responsible conductance for purines released from inhibitory enteric motor neurons.9 Activation of SK channels in PDGFRα+ cells induces hyperpolarization.8
Our previous study has shown that the density of ICC distribution was different from proximal and distal colons. ICCs are highly distributed in the proximal colon than in the distal colon. In contrast, density of PDGFRα+ cells in the distal colon is higher compared to the proximal colon.10 However, there is no clear description on the role of this different cellular distribution on colonic motility including colonic migrating motor complex (CMMC).
The mechanisms of colonic dysmotility in partial colon obstruction (PCO) is not fully clarified. In previous studies, obstructed animal models and patients with idiopathic chronic pseudo-obstruction showed a decreased density of the ICCs.11–14 In the study of murine proximal colon, PCO did not affect ICC density on days 1 and 3. However, the ICC network was disrupted on day 7 of PCO.15 These data suggested that partial loss of ICC may be associated with colonic dysmotility. However, besides studies of morphological changes, the mechanisms of functional alteration in the PCO colon have not been studied.
In the present study, we established a murine PCO model to investigate the changes in protein expression of Kit, platelet-derived growth factor receptor-α (Pdgfra), Ano1, and SK3 channels using western blotting. We also examined the functional alteration in PCO mice by measuring colonic CMMCs and isometric force measurements.
Materials and Methods
Ethics Statement
This study was performed in compliance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Science and Technology Commission of P.R.C. (STCC Publication No. 2, revised 1988). The protocol followed the instructions by the Committee on the Ethics of Animal Experiments (Permit No. Hu 686-2009).
Animal Model
Male specific pathogen-free (SPF) C57BL/6 mice aged 5–6 weeks with body weights of 20 ± 4 g were used (Shanghai SLAC Laboratory Animal Co, Shanghai, China). The mice were housed under 22–24°C (12 hour-light and 12-hour dark cycle), with free access to food and water. PCO mouse model was prepared by the following procedures. The mice were fasted for 6 hours before surgery, and then were anesthetized with 2% isoflurane inhalation. A midline laparotomy was performed on the mice. A silicon ring (4 mm × 4 mm, 3 mm internal diameter) was applied to the distal colon 3 cm proximal to the end of the murine colon. After surgery, all efforts were made to minimize suffering, including analgesic medications and put the mice in a 35°C water bath. The mice were kept for 7 days until euthanasia. Monitoring was kept for weight, fecal pellets, and mental condition of the postoperative mice every day. Experiments were performed with the murine colons 7 days after surgery. The mice were euthanized with 2% isoflurane and sacrificed by cervical dislocation. The colon tissue from the ileocecal valve to the distal colon of obstruction was removed and pinned to the base of a Sylgard silicone elastomer dish containing oxygenated (95% O2 and 5% CO2) Krebs solution.
Western Blot Analysis
Protein samples were harvested from colonic smooth muscle and homogenized in ice-cold RIPA buffer (1:10; P0013; Beyotime Chemical Co, Jiangsu, China) and 1% protease inhibitor cocktail (P2714; Sigma Aldrich, County Wicklow, Ireland). The homogenates were centrifuged at 12000 rpm for 15 minutes at 4°C. The supernatant collection and protein extraction were described previously.9 In brief, primary antibodies were incubated overnight at 4°C and secondary antibodies at room temperature for 2 hours. An anti-Pdgfrα antibody (1:1000; 3174; Cell Signaling Technology, Danvers, MA, USA), anti-SK3 antibody (1:500; ab28631; Abcam, Cambridge, UK), anti-Kit antibody (1:1000; 3074; Cell Signaling Technology), anti-Ano1 antibody (1:1000; ab191040; Abcam), anti-glyceraldehyde-3-phosphate dehydrogenas antibody (1:1000; GB11002; Servicebio, Wuhan, China), and HRP-linked anti-rabbit antibody (1:1000; 7074; Cell Signaling Technology) were used.
CMMC Experiments
Mice were sacrificed with overdose isoflurane inhalation followed by cervical dislocation. The whole colon was extracted from the body and carefully dissected to remove mesentery and fat tissues. All fecal materials were carefully expelled with repeated injection of Krebs solution. A glass capillary tube was inserted through the empty lumen to fix the colon segment to the dish floor. The colon segment was perfused with warm Krebs solution (37°C, 5% CO2 and 95% O2) and stabilized for 30–40 minutes to recover colonic contraction activity. Silk thread (USP 5/0) was applied to proximal and distal parts of the colon to connect force transducers. The CMMC activity was recorded by an isometric force transducer (RM6240C; Chengdu Instrument Factory, Chengdu, China).
Spontaneous Contractions of Smooth Muscle
The mice were sacrificed as described above. The mucosa and submucosa layers were removed by fine dissecting scissors under a microscope. The smooth muscle strips (8 mm × 2 mm) were cut along the circular axis. A force transducer (RM6240C; Chengdu) was used for contractions along the longitudinal axis in 10 mL organ baths with 37°C oxygenated (95% O2 and 5% CO2) Krebs solution. Krebs solution was recycled every 20 minutes. The area under the curve (AUC) was calculated by adjustment of resting tension to baseline in control traces. Spontaneous contractions for 2 minute recordings and CMMC for 10 minutes recordings were used for analysis. AUC was normalized to control to compare with effects of drugs.
Solutions and Drugs
The Krebs solution: NaCl, 118.5 mM; KCl, 4.5 mM; MgCl2, 1.2 mM; NaHCO3, 23.8 mM; KH2PO4, 1.2 mM; glucose 11.0 mM; and CaCl2, 2.4 mM. NG-Nitro-l-arginine methyl ester hydrochloride (L-NAME), 5-Nitro-2-(3-phenylpropylamino) benzoic acid (NPPB), Apamin and CyPPA were purchased from Tocris Bioscience (Ellisville, MO, USA).
Statistical Methods
Data are shown as the mean ± SEM. Student’s unpaired t test was carried out to compare statistical significance in groups. Differences at P < 0.05 level were considered statistically significant. N values represent the number of animals used in the experiments. Data analysis was performed with GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA).
Results
Anatomic and Histological Changes in PCO Mice
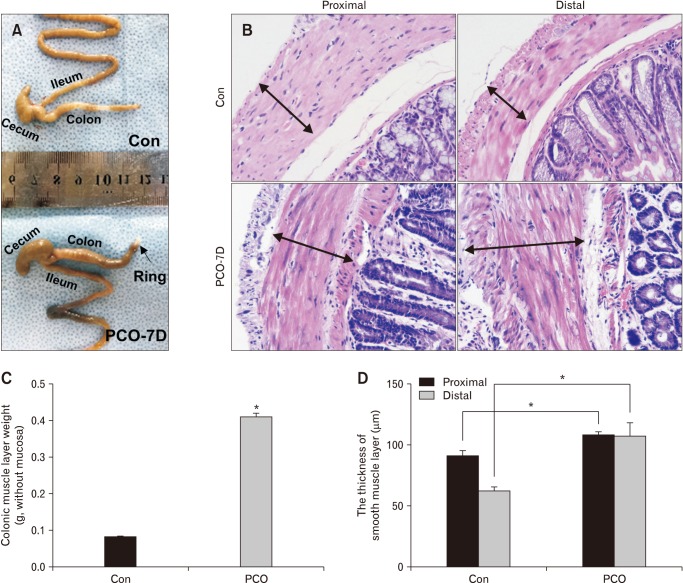
Distended abdomen was detected on the 7th day after surgery, with the absence of fecal pellet production. The colon was obviously distended, and large amounts of feces accumulated above the ring (Fig. 1A). The colon tissue in the proximal dilated segment (> 2 cm) and the distal segment (< 2 cm) from the inserted ring was dissected and analyzed by hematoxylin & eosin (HE) staining (Fig. 1B), and the results showed that the smooth muscle layer was hypertrophied. The weight of the colonic smooth muscle layers in PCO mice increased significantly after removing the mucosa (Fig. 1C). The average thickness of the smooth muscle layer in control and PCO mice was significantly different (Fig. 1D).
Figure 1.
Surgically induced partial colon obstruction (PCO) in a mouse model. (A) Pictures showing the anatomic changes in control (Con) and PCO mice. (B, D) Comparison of the thickness of the smooth muscle layer between control and PCO mice by H&E staining (×200 magnification) and statistical analysis (*P < 0.05, n = 7). (C) Summarized data showing the colonic muscle layer weight in control and PCO mice (*P < 0.05, n = 7).
The Alteration of ICCs and Ano1 in Hypertrophic Colonic Smooth Muscle Layers
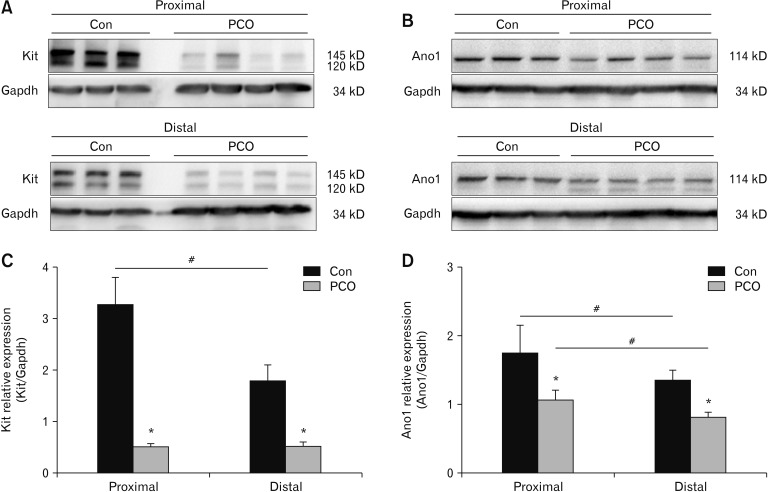
To determine the remodeling of ICCs due to PCO, western blot approach was used to examine the expression of Kit and Ano1. We explored the density of Kit and Ano1 protein expression in the colonic smooth muscle tissues. Kit expression was higher in the proximal colon than in the distal colon (Fig. 2A). In the comparison of Kit in PCO and control colons, Kit in PCO was decreased to 0.48 ± 0.12 compared to control mice (3.24 ± 0.45) in the proximal colon and to 0.61 ± 0.14 compared to control mice (1.79 ± 0.26) in the distal colon (*P < 0.05, n = 6; Fig. 2A and 2C). Similarly, Ano1 expression was slightly lower in the distal colon compared to the proximal colon. In PCO colon, Ano1 was downregulated to 1.06 ± 0.20 compared to control (1.73 ± 0.35) in the proximal colon and to 0.85 ± 0.11 compared to control (1.34 ± 0.15) in the distal colon (*P < 0.05, n = 6; Fig. 2B and 2D).
Figure 2.
Expression of Kit and Ano1 in the colonic muscle layers of control (Con) and partial colon obstruction (PCO) mice. Western blot analysis of Kit (A) and Ano1 (B) in control and PCO mice. (C, D) The data were analyzed using densitometric quantification by Quantity One (% glyceraldehyde-3-phosphate dehydrogenase [Gapdh], normalized to data from control mice; n = 6, *P < 0.05, PCO vs control, #P < 0.05 proximal vs distal).
The Responses of Colonic Smooth Muscle to L-NAME and NPPB
To observe the remodeling of ICCs and Ano1 channels in control and PCO colons, the effects of the NO synthase inhibitor L-NAME and the Ano1 channel inhibitor NPPB on CMMC were examined using whole colons and muscle strips.
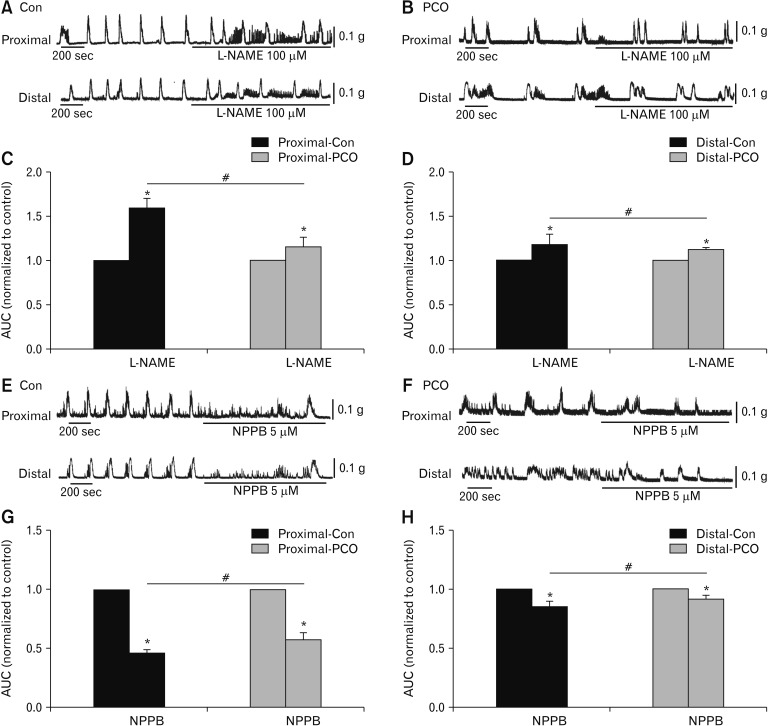
L-NAME (100 μM) significantly enhanced the area under the curve (AUC) values of CMMCs. L-NAME increased the AUC of the proximal colon increased to 158.7 ± 6.5% in control mice and 113.6 ± 6.5% in the PCO mice (*P < 0.05, n = 5; Fig. 3A–C). In the distal colon, the corresponding AUC values also increased to 114.6 ± 7.6% and 111.2 ± 2.0% (*P < 0.05, n = 5; Figure 3A, B and D). However, the effectiveness of L-NAME was significantly decreased in PCO compared to control (#P < 0.05 in proximal colons, #P < 0.05 in distal colons; Fig. 3C and 3D). To evaluate the function of Ano1 channel, NPPB (5 μM) was applied. The colonic transmission in the proximal colon was reduced to 46.2 ± 1.8% in the control mice and 57.5 ± 3.4% in the PCO mice (*P < 0.05, n = 5; Fig. 3E–G). In the distal colon, the CMMC transmission was reduced to 84.2 ± 2.9% and 91.4 ± 2.1%, respectively (*P < 0.05, n = 5; Fig. 3E, 3F, and 3H). NPPB showed less effect on CMMCs in PCO compared to control (#P < 0.05 in proximal colons, #P < 0.05 in distal colons; Fig. 3G and 3H).
Figure 3.
Effects of inhibitors of nitric oxide synthase (NOS) and Ca2+-activated Cl− channel (Ano1) on colonic migrating motor complexes (CMMCs) in the colonic muscles of control (Con) and partial colon obstruction (PCO) mice. (A, B) The inhibitory effects of NG-Nitro-l-argi-nine methyl ester hydrochloride (L-NAME; 100 μM) on CMMCs in the proximal and distal colons of both control and PCO mice. (C, D) The area under the curve (AUC) were normalized to the control (before the application of L-NAME) (n = 5; *P < 0.05, L-NAME vs control; #P < 0.05, PCO vs control mice). (E, F) Response of CMMCs to 5-Nitro-2-(3-phenylpropylamino) benzoic acid (NPPB; 5 μM) in the proximal and distal colons in both control and PCO mice. (G, H) Summary of the response of CMMCs to NPPB in colonic muscles of control and PCO mice. The data were normalized to the control value (before the application of NPPB) (n = 5; *P < 0.05, NPPB vs control; #P < 0.05, PCO vs control mice).
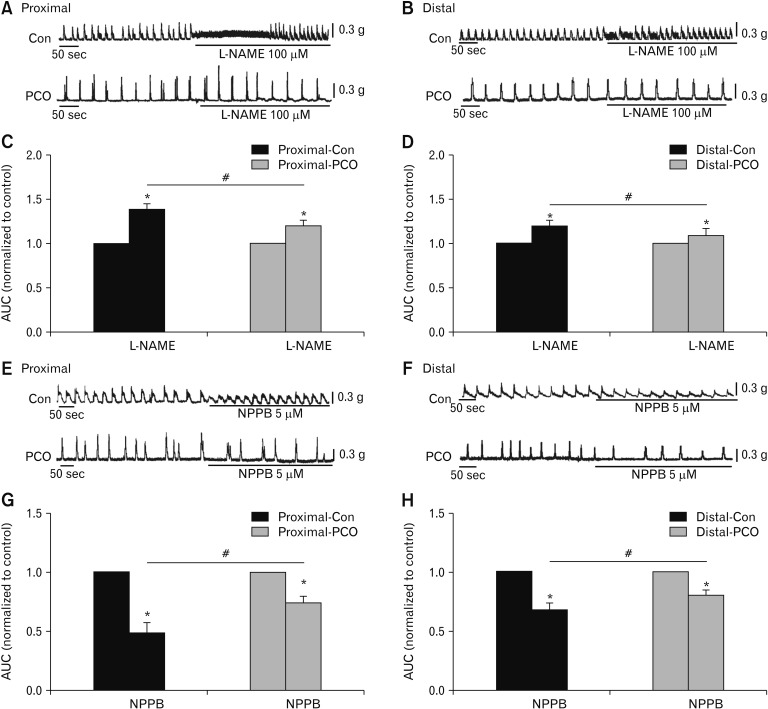
The effects of L-NAME and an Ano1 blocker to colonic muscle spontaneous contraction were investigated. L-NAME remarkably strengthened spontaneous contractions of the proximal colon in controls to 137.9 ± 3.9% (*P < 0.05, n = 5; Fig. 4A and 4C), and in PCO mice to 119.9 ± 5.5% (*P < 0.05, n = 5; Fig. 4A and 4C). In the distal colon, AUC after L-NAME were also enhanced to 119.5 ± 4.1% (*P 0.05, n = 5; Fig. 4B and 4D) in control and 110.2 ± 10.7% (*P < 0.05, n = 5; Fig. 4B and 4D) in PCO, respectively. However, the effect of L-NAME was significantly decreased in PCO mice compared to the control mice (#P < 0.05 in proximal colons, #P < 0.05 in distal colons; Fig. 4C and 4D). NPPB decreased the AUC of spontaneous contractions to 48.9 ± 6.7% vs 67.0 ± 9.5% in the proximal colon (control vs PCO mice, n = 5, P < 0.05; Fig. 4E and 4G) and 66.5 ± 3.9% vs 80.0 ± 4.1% in the distal colon (control vs PCO mice, n = 5, P < 0.05; Fig. 4F and 4H). However, the inhibitory effects of NPPB were decreased in the PCO murine colon smooth muscle compared to control mice (#P < 0.05 in proximal colons, #P < 0.05 in distal colons; Fig. 4G and 4H). Therefore, it was indicated that NO contributed significantly to the regulation of CMMC frequency, and ICCs and Ano1 channels may be downregulated in PCO mice in both the proximal and distal colons.
Figure 4.
Contractile responses to inhibitors of nitric oxide synthase (NOS) and Ca2+-activated Cl− channel (Ano1) in the colonic muscles of control (Con) and partial colon obstruction (PCO) mice. (A, B) The inhibitory effects of NG-Nitro-l-arginine methyl ester hydrochloride (L-NAME; 100 μM) on spontaneous contractions in the proximal and distal colons of both control and PCO mice. (C, D) Summary data shows the normalized area under the curve (AUC) in control and after the application of L-NAME (n = 5; *P < 0.05, control vs L-NAME; #P < 0.05, control vs PCO mice). (E, F) Response of colonic smooth muscle contractions to 5-Nitro-2-(3-phenylpropylamino) benzoic acid (NPPB; 5 μM) in the proximal and distal colon in both control and PCO mice. (G, H) Summary of the contractile responses to NPPB as indicated by the AUC in colonic muscles of control and PCO mice. The data were normalized to the control value (before the application of NPPB) (n = 5; *P < 0.05, NPPB vs control; #P < 0.05, PCO vs control mice).
The Alteration of PDGFRα+ Cells and SK3 in Hypertrophic Colonic Smooth Muscles
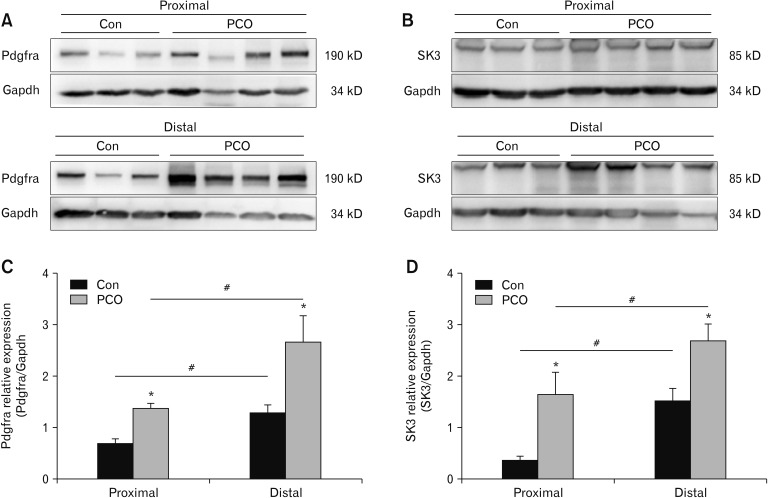
The western blot showed that the expression levels of Pdgfra protein were much higher in the PCO colon compared to those in control mice (*P < 0.05, n = 6; Fig. 5A and 5C). In the control group, the relative expression rates were 0.68 ± 0.09 in the proximal colon and 1.28 ± 0.93 in the distal colon (#P < 0.05, n = 6; Fig. 5A and 5C). In the PCO group, the relative expression rates were 1.35 ± 0.08 in the proximal segment and 2.50 ± 0.25 in the distal segment (#P < 0.05, n = 6; Fig. 5A and 5C). The expression of SK3 protein was higher in the PCO murine proximal and distal colon compared with those in control mice (*P < 0.05, n = 6; Fig. 5B and 5D). These results directly show that PCO mice have less density of ICCs and higher density of PDGFRα+ cells in both proximal and distal colons.
Figure 5.
Western blot analysis of platelet-derived growth factor receptor-α (Pdgfra; A) and small conductance Ca2+-activated K+ channel 3 (SK3; B) in control (Con) and partial colon obstruction (PCO) mice. (C, D) The data were analyzed using densitometric quantification by Quantity One (% glyceraldehyde-3-phosphate dehydrogenase [Gapdh], normalized to data from control mice; n = 6, *P < 0.05 vs control, #P < 0.05 proximal vs distal).
The Effect of Apamin and CyPPA on Colonic Smooth Muscle
To investigate the functional alteration of SK3 channels in PDGFRα+ cells, the effects of the SK agonist, CyPPA and antagonist, apamin on CMMC and contractions of colonic muscle strips were observed.
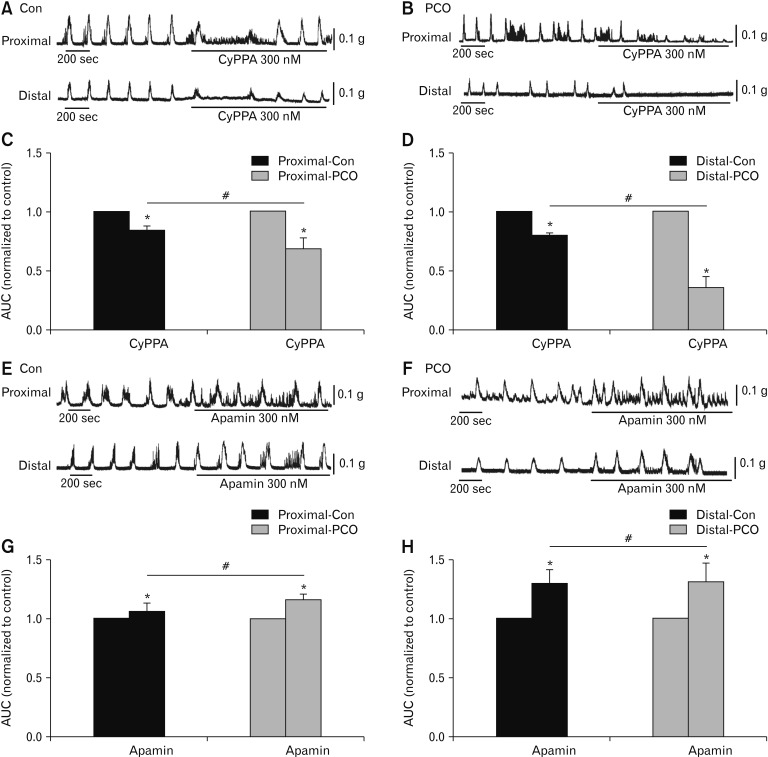
In the proximal colon, CyPPA (300 nM) reduced the AUC to 91.9 ± 2.7% in control and 88.6 ± 0.8% in PCO mice, respectively. These effects were significantly different in control and PCO (*P < 0.05, n = 5; Fig. 6A–C). In the distal colon, CyPPA also decreased the AUC to 79.7 ± 2.2% in control and to 36.1 ± 5.3% in PCO mice. These effects were also different in control and PCO (*P < 0.05, n = 5; Fig. 6A, 6B, and 6D). The relaxation effect of the SK agonist was more obvious in the PCO murine colon (#P < 0.05 in proximal colons, #P < 0.05 in distal colons, Fig. 6C and 6D). Apamin (300 nM) enhanced the AUC of CMMC activity to 106.2 ± 7.7% in control and 116.9 ± 3.6% in PCO of the proximal colon (*P < 0.05, n = 5; Fig. 6E–G), and to 120.7 ± 3.6% in control and 128.3 ± 9.3% in PCO of the distal colon (*P < 0.05, n = 5; Fig. 6E, 6F, and 6H). The effects of apamin were more remarkable in the PCO mice (#P < 0.05 in proximal colons, #P < 0.05 in distal colons; Fig. 6G and 6H).
Figure 6.
Response of colonic migrating motor complexes (CMMCs) to a small conductance Ca2+-activated K+ channel (SK) agonist and antagonist in the colonic muscles of control (Con) and partial colon obstruction (PCO) mice. (A, B) The inhibitory effects of SK agonist, CyPPA (300 nM), on CMMCs in the proximal and distal colons in both control and PCO mice. (C, D) Summary of the area under the curve (AUC) data shows the normalized values to the control (before the application of CyPPA) (n = 5; *P < 0.05, CyPPA vs control; #P < 0.05, PCO vs control mice). (E, F) Response of CMMCs to apamin (300 nM) in the proximal and distal colons in both control and PCO mice. (G, H) Summary of the CMMCs to apamin in colonic muscles from control and PCO mice. The data were normalized to the control value (before the application of apamin) (n = 5; *P < 0.05, apamin vs control; #P < 0.05, PCO vs control mice).
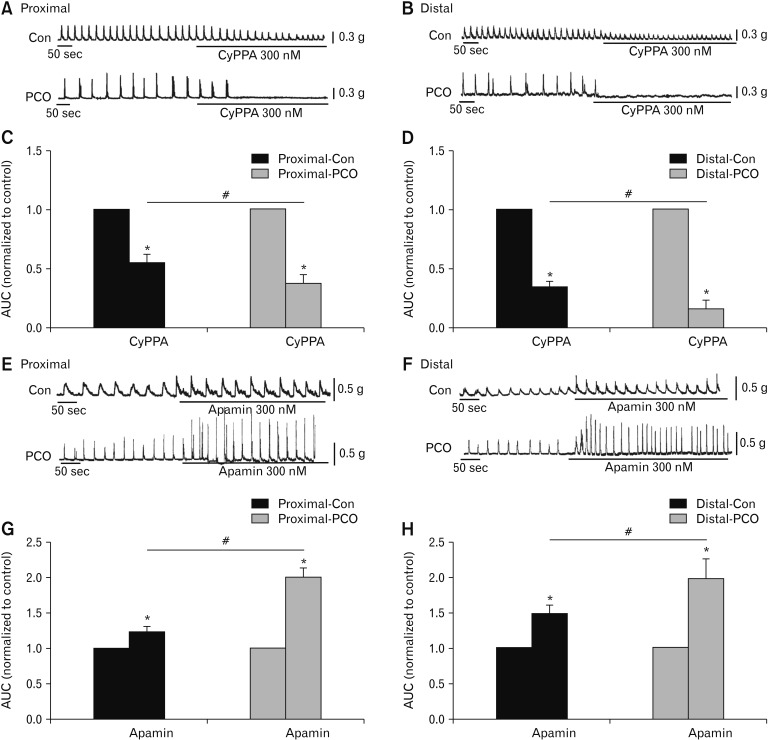
Isometric force measurements using colonic muscle strips were also performed to test effectiveness of CyPPA and apamin. CyPPA reduced the AUC of proximal colonic smooth muscle to 55.2 ± 5.7% in control and 37.8 ± 8.0% in PCO (*P < 0.05, n = 5; Fig. 7A and 7C), respectively. In the distal colon, the AUC by CyPPA treatment were decreased to 33.3 ± 3.2% in control and 16.3 ± 7.9% in PCO (*P < 0.05, n = 5; Fig. 7B and 7D). The PCO colonic strips were more sensitive to CyPPA compared with control mice (#P < 0.05 in proximal colons, #P < 0.05 in distal colons; Fig. 7C and 7D). In control muscle strips, apamin enhanced to 123.9 ± 5.9% in the proximal colon (*P < 0.05, n = 5; Fig. 7E and 7G) and 147.5 ± 6.8% in the distal colon (*P < 0.05, n = 5; Fig. 7F and 7H). Interestingly, in the PCO colon, apamin dramatically increased the AUC to 200.3 ± 10.8% in proximal colon (*P < 0.05, n = 5; Fig. 7E and 7G) and 196.8 ± 29.2% in the distal colon (*P < 0.05, n = 5; Fig. 7F and H). Murine colonic smooth muscle in PCO showed greater effectiveness of apamin (#P < 0.05 in proximal colons, #P < 0.05 in distal colons; Fig. 7G and 7H). According to these results, we suggest that PDGFRα+ cells and SK3 channels may be upregulated in PCO mice in both the proximal and distal colons.
Figure 7.
Contractile responses to the small conductance Ca2+-activated K+ channel (SK) agonist and antagonist in the colonic muscles of control (Con) and partial colon obstruction (PCO) mice. (A, B) The inhibitory effects of SK agonist, CyPPA (300 nM), on spontaneous contractions in the proximal and distal colons in both control and PCO mice. (C, D) Summary of the area under the curve (AUC) in control and PCO. The data were normalized to the control (before the application of CyPPA) (n = 5; *P < 0.05, CyPPA vs control; #P < 0.05, PCO vs control mice). (E, F) Response of colonic smooth muscle contractions to apamin (300 nM) in the proximal and distal colon in both control and PCO mice. (G, H) Summary of the contractile responses to apamin in the colonic muscles of control and PCO mice. The data were normalized to the control value (before the application of apamin) (n = 5; *P < 0.05, apamin vs control; #P < 0.05, PCO vs control mice).
Discussion
PCO occurs on account of mechanical or functional obstruction.16,17 Mechanical obstruction happens due to a block of the intraluminal passage including adhesions, hernias, carcinoma and diverticulitis.18,19 Functional obstruction could be due to neuromuscular disorders such as Hirschsprung’s disease.20–22 Mechanical and functional obstruction induces distended proximal colon in the obstruction site with accumulation of the feces and gas.23 The obstructed segments displayed altered motility and increased thickness of the smooth muscle layer.24,25 Besides the morphological changes in PCO, the pathophysiological mechanisms of colonic dysmotility in PCO are not known.18,19
A reliable PCO model had been established by using a silicon band around the distal colon wall through the mesentery in literature.26–28 In the present experiments, the silicon ring was placed at the end of distal colon. Feces were accumulated at the distal end of the colon from the obstructed lesion due to difficulty of feces to pass through the obstructed colonic segment. After surgery, the mice gradually developed abdominal distention. The mice were sacrificed 7 days after operation. H&E staining showed muscle layer hypertrophy (Fig. 1). This PCO model was used to investigate the mechanism of colonic dysmotility.
Generation of CMMC is due to activation of the enteric nervous system that drives fecal pellets in an aboral direction.29 Excitatory and inhibitory neurotransmitters affect the activity of ICC and induce CMMC via smooth muscle contractions in the intact colonic segment. In previous reports, a partial intestinal obstruction mouse model showed ICC remodeling.30,31 Loss of ICC could disrupt CMMC. The present data showed significant changes in the frequency of CMMC (decreased AUC) in PCO compared to controls.
To determine the relationship between ICCs and colonic dysmotility in PCO mice, we examined the expression of Kit and Ano1 proteins. Kit and Ano1 decreased significantly in the proximal (> 2 cm from oral to obstructed lesion) and distal segment (2 cm from oral to obstructed lesion) in PCO mice. In previous reports, loss or disruption of ICC networks was reported in an obstruction model.14 Furthermore, when ICCs in the small intestine were disrupted by the obstruction, the loss of pacemaker electrical activity and responses to neurotransmitters were observed.11 Ano1 channels in ICC are the main conductance to generate pacemaker activity.32 In the present study, we used NPPB, an Ano1 blocker to test functional changes of Ano1 in PCO. Colonic muscle strips were used for measuring spontaneous contractions. NPPB showed less effect in PCO than in control contractions. Similar responses were observed on CMMCs. These data suggest that PCO exhibited functional downregulation of Ano1 channels in ICC. The underlying mechanisms of downregulation of Kit and Ano1 in PCO are unknown.
ICC-IM in the colon are the main target cells of NO. NO is one dominating inhibitory neurotransmitter in the colon.33,34 We tested the effect of the NO synthase inhibitor on the spontaneous contractions of smooth muscle and CMMCs in control and PCO mice. The results showed that contractions of colonic smooth muscle were enhanced after the addition of L-NAME in control colons. The effect of L-NAME was less sensitive in PCO compared to controls. This result could be due to downregulation of nNOS shown in colonic pseudo-obstruction,35 and downregulation of Kit based on the present findings.
PDGFRα+ cells express SK3 protein. Inhibitory neurotransmitters, mainly purines bind to G-protein coupled P2Y1 receptors in PDGFRα+ cells,4,9,36 increase intracellular Ca2+, activate SK3 channels, and induce hyperpolarization.37,38 In the present study, western blot displayed that the Pdgfra and SK3 proteins were significantly upregulated in PCO colon compared with those of control mice. We also tested the effects of SK channel agonist and antagonist on the spontaneous contractions of muscle strips from the proximal and distal colons in controls and PCO. The results showed that the SK agonist, CyPPA significantly decreased contractions in control colonic muscles. PCO colons were more sensitive to CyPPA compared to responses in control colonic muscles. Apamin, a SK antagonist increased the AUC of spontaneous contractions in controls. Moreover, apamin sensitivity was higher in PCO than in control colons. In CMMC, the effects of apamin were also significantly stronger in the PCO mice than in the normal control colons. Our data suggest that the upregulation of the Pdgfra and SK3 channels in the PCO colon induced significant inhibitory effects by SK agonist and excitatory effects by SK antagonist on muscle contractions and CMMCs. Mechanisms of gain or upregulation of Pdgfra and SK3 are not known. Understanding mechanisms of upregulation of these proteins will be an interesting future project.
In summary, PCO mice displayed different patterns of smooth muscle contractions and CMMCs. PCO mice exhibited colonic dysmotility due to downregulation of Kit and Ano1 channels, and upregulation of Pdgfra and SK3 channels. These data were supported by functional analysis. Spontaneous contractions and CMMCs were less sensitive to nNOS and Ano1 inhibitors but more sensitive to SK modulators in PCO colons. In conclusion, PCO leads to colonic dysmotility due to density changes in ICC and PDGFRα+ cells and their subsequent changes in excitability.
Footnotes
Conflicts of interest: None.
Author contributions: Jie Chen was responsible for conception and design of the experiments; Xu Huang, Hongli Lu, and Wenxie Xu were responsible for the collection, analysis, and interpretation of data; Qianqian Wang and Jingyu Zang performed specific experimental tasks; Qianqian Wang drafted the article; and Jie Chen revised the manuscript critically for important intellectual content. All authors signed and approved the final submission.
Financial support: This study was supported by grants from Science and Technology Planning Project of Jiaxing City (No. 2018AY32019); Zhejiang Provincial Natural Science Foundation of China (No. LY20C11009); Research Fund for Lin He’s Academician Workstation of New Medicine and Clinical Translation; and Science and Research Fund of Xinhua Hospital (No. 18YJ01-XH2406).
References
- 1.Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/W(nu) mouse small intestine. J Auton Nerv Syst. 2000;80:142–147. doi: 10.1016/S0165-1838(00)00089-8. [DOI] [PubMed] [Google Scholar]
- 2.Iino S, Horiguchi K, Horiguchi S, Nojyo Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol. 2009;131:691–702. doi: 10.1007/s00418-009-0580-6. [DOI] [PubMed] [Google Scholar]
- 3.Koh SD, Ward SM, Sanders KM. Ionic conductances regulating the excitability of colonic smooth muscles. Neurogastroenterol Motil. 2012;24:705–718. doi: 10.1111/j.1365-2982.2012.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung TS, Hwang SJ, Koh SD, et al. The cells and conductance mediating cholinergic neurotransmission in the murine proximal stomach. J Physiol. 2018;596:1549–1574. doi: 10.1113/JP275478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blair PJ, Rhee PL, Sanders KM, Ward SM. The significance of interstitial cells in neurogastroenterology. J Neurogastroenterol Motil. 2014;20:294–317. doi: 10.5056/jnm14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol. 2006;576:675–682. doi: 10.1113/jphysiol.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurahashi M, Zheng H, Dwyer L, Ward SM, Koh SD, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589(Pt 3):697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiménez M. Platelet-derived growth factor receptor-alpha-positive cells: new players in nerve-mediated purinergic responses in the colon. J Physiol. 2015;593:1765–1766. doi: 10.1113/JP270259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh SD, Rhee PL. Ionic conductance(s) in response to post-junctional potentials. J Neurogastroenterol Motil. 2013;19:426–432. doi: 10.5056/jnm.2013.19.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu C, Huang X, Lu HL, et al. Different distributions of interstitial cells of Cajal and platelet-derived growth factor receptor-alpha positive cells in colonic smooth muscle cell/interstitial cell of Cajal/platelet-derived growth factor receptor-alpha positive cell syncytium in mice. World J Gastroenterol. 2018;24:4989–5004. doi: 10.3748/wjg.v24.i44.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang IY, Glasgow NJ, Takayama I, Horiguchi K, Sanders KM, Ward SM. Loss of interstitial cells of Cajal and development of electrical dysfunction in murine small bowel obstruction. J Physiol. 2001;536(Pt 2):555–568. doi: 10.1111/j.1469-7793.2001.0555c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fintl C, Hudson NP, Mayhew IG, Edwards GB, Proudman CJ, Pearson GT. Interstitial cells of Cajal (ICC) in equine colic: an immunohistochemical study of horses with obstructive disorders of the small and large intestines. Equine Vet J. 2004;36:474–479. doi: 10.2746/0425164044877314. [DOI] [PubMed] [Google Scholar]
- 13.Wu B, Liu L, Gao H, et al. Distribution of interstitial cells of Cajal in Meriones unguiculatus and alterations in the development of incomplete intestinal obstruction. Histol Histopathol. 2013;28:1567–1575. doi: 10.14670/HH-28.1567. [DOI] [PubMed] [Google Scholar]
- 14.Becheanu G, Manuc M, Dumbravă M, Herlea V, Hortopan M, Costache M. The evaluation of interstitial Cajal cells distribution in non-tumoral colon disorders. Rom J Morphol Embryol. 2008;49:351–355. [PubMed] [Google Scholar]
- 15.Wu CC, Lin YM, Gao J, Winston JH, Cheng LK, Shi XZ. Are interstitial cells of Cajal involved in mechanical stress-induced gene expression and impairment of smooth muscle contractility in bowel obstruction? PLoS One. 2013;8:e76222. doi: 10.1371/journal.pone.0076222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Summers RW, Lu CC. Approach to the patient with ileus and obstruction. In: Yamada T, Alpers DH, Laine L, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 1st ed. Vol. 1. Philadelphia: Saunders; 1999. pp. 842–858. [Google Scholar]
- 17.Welch JP. Bowel obstruction: differential diagnosis and clinical management. 1st ed. Philadelphia: Saunders; 1990. pp. 59–95. [Google Scholar]
- 18.Cappell MS, Batke M. Mechanical obstruction of the small bowel and colon. Med Clin North Am. 2008;92:575–597. doi: 10.1016/j.mcna.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Rami Reddy SR, Cappell MS. A systematic review of the clinical presentation, diagnosis, and treatment of small bowel obstruction. Curr Gastroenterol Rep. 2017;19:28. doi: 10.1007/s11894-017-0566-9. [DOI] [PubMed] [Google Scholar]
- 20.De Giorgio R, Cogliandro RF, Barbara G, Corinaldesi R, Stanghellini V. Chronic intestinal pseudo-obstruction: clinical features, diagnosis, and therapy. Gastroenterol Clin North Am. 2011;40:787–807. doi: 10.1016/j.gtc.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Wells CI, O’Grady G, Bissett IP. Acute colonic pseudo-obstruction: a systematic review of aetiology and mechanisms. World J Gastroenterol. 2017;23:5634–5644. doi: 10.3748/wjg.v23.i30.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heuckeroth RO. Hirschsprung disease - integrating basic science and clinical medicine to improve outcomes. Nat Rev Gastroenterol Hepatol. 2018;15:152–167. doi: 10.1038/nrgastro.2017.149. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs DO. Acute intestinal obstruction. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, editors. Harrison’s principles of internal medicine. 20th ed. Vol. 10. New York: McGraw-Hill; 2014. p. 323. [Google Scholar]
- 24.Bertoni S, Gabella G, Ghizzardi P, et al. Motor response of rat hypertrophic intestine following chronic obstruction. Neurogastroenterol Motil. 2004;16:365–374. doi: 10.1111/j.1365-2982.2004.00510.x. [DOI] [PubMed] [Google Scholar]
- 25.Won KJ, Suzuki T, Hori M, Ozaki H. Motility disorder in experimentally obstructed intestine: relationship between muscularis inflammation and disruption of the ICC network. Neurogastroenterol Motil. 2006;18:53–61. doi: 10.1111/j.1365-2982.2005.00718.x. [DOI] [PubMed] [Google Scholar]
- 26.Shi XZ, Lin YM, Powell DW, Sarna SK. Pathophysiology of motility dysfunction in bowel obstruction: role of stretch induced COX-2. Am J Physiol Gastrointest Liver Physiol. 2011;300:G99–G108. doi: 10.1152/ajpgi.00379.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin YM, Sarna SK, Shi XZ. Prophylactic and therapeutic benefits of COX-2 inhibitor on motility dysfunction in bowel obstruction: roles of PGE2 and EP receptors. Am J Physiol Gastrointest Liver Physiol. 2012;302:G267–G275. doi: 10.1152/ajpgi.00326.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin YM, Fu Y, Winston J, et al. Pathogenesis of abdominal pain in bowel obstruction: role of mechanical stress-induced upregulation of nerve growth factor in gut smooth muscle cells. Pain. 2012;158:583–592. doi: 10.1097/j.pain.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heredia DJ, Dickson EJ, Bayguinov PO, Henning GW, Smith TK. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology. 2009;136:1328–1338. doi: 10.1053/j.gastro.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu DH, Huang X, Guo X, et al. Voltage dependent potassium channel remodeling in murine intestinal smooth muscle hypertrophy induced by partial obstruction. PLoS One. 2014;9:e86109. doi: 10.1371/journal.pone.0086109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Zhao J, Chen P, Nakaguchi T, Grundy D, Gregersen H. Interdependency between mechanical parameters and afferent nerve discharge in hypertrophic intestine of rats. Am J Physiol Gastrointest Liver Physiol. 2016;310:G376–G386. doi: 10.1152/ajpgi.00192.2015. [DOI] [PubMed] [Google Scholar]
- 32.Zhu MH, Kim TW, Ro S, et al. A Ca2+-activated Cl− conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009;587(Pt 20):4905–4918. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki H, Ward SM, Bayguinov YR, Edwards FR, Hirst GD. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J Physiol. 2003;546:751–763. doi: 10.1113/jphysiol.2002.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward SM, Sanders KM. Physiology and pathophysiology of the interstitial cell of Cajal: from bench to bedside. I. Functional development and plasticity of interstitial cells of Cajal networks. Am J Physiol Gastrointest Liver Physiol. 2001;281:G602–G611. doi: 10.1152/ajpgi.2001.281.3.G602. [DOI] [PubMed] [Google Scholar]
- 35.Do YS, Myung SJ, Kwak SY, et al. Molecular and cellular characteristics of the colonic pseudo-obstruction in patients with intractable constipation. J Neurogastroenterol Motil. 2015;21:560–570. doi: 10.5056/jnm15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song NN, Lu HL, Lu C, et al. Diabetes-induced colonic slow transit mediated by the up-regulation of PDGFRα+ cells/SK3 in streptozotocin-induced diabetic mice. Neurogastroenterol Motil. doi: 10.1111/nmo.13326. Published Online First: 9 Mar 2018. [DOI] [PubMed] [Google Scholar]
- 37.Baker SA, Hennig GW, Salter AK, Kurahashi M, Ward SM, Sanders KM. Distribution and Ca2+ signalling of fibroblast-like (PDGFR+) cells in the murine gastric fundus. J Physiol. 2013;591:6193–6208. doi: 10.1113/jphysiol.2013.264747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. 2014;94:859–907. doi: 10.1152/physrev.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]