Figure 2. Library properties.
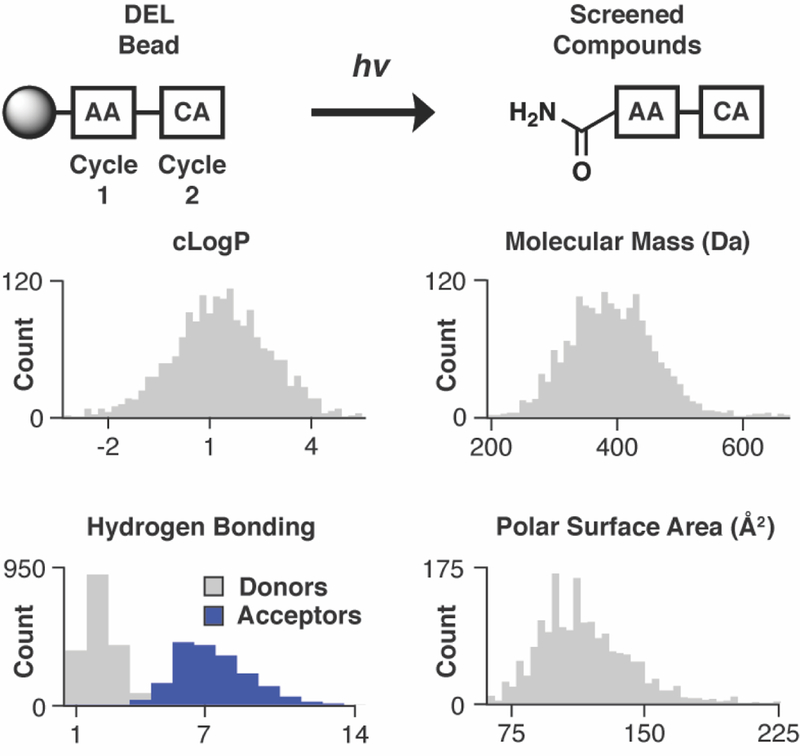
The library was generated with two cycles of acylation chemistry with amino acid (AA) and carboxylic acid (CA) monomers, respectively. Photochemical cleavage releases compound from the library bead as a primary amide. Physicochemical property analysis of photochemically cleaved compounds included hydrophobicity (cLogP), molar mass, hydrogen bond donors/acceptors, and polar surface area. Most compounds analyzed (1,738/1,766) are ‘drug-like’.
