Abstract
Background
Complicated intra-abdominal infections (cIAIs) result in significant morbidity, mortality, and cost. Carbapenem-resistant sepsis has increased dramatically in the last decade, resulting in infections that are difficult to treat and associated with high mortality rates. To prevent further antibacterial resistance, it is necessary to use carbapenem selectively. The objective of this study was to compare the effectiveness and safety of carbapenems vs alternative β-lactam monotherapy or combination therapy for the treatment of cIAIs.
Methods
The PubMed, Embase, Medline (via Ovid SP), and Cochrane library databases were systematically searched. We included randomized controlled trials (RCTs) comparing carbapenems vs alternative β-lactam monotherapy or combination therapy for the treatment of cIAIs.
Results
Twenty-two studies involving 7720 participants were included in the analysis. There were no differences in clinical treatment success (odds ratio [OR], 0.86; 95% confidence interval [CI], 0.71–1.05; I2 = 35%), microbiological treatment success (OR, 0.88; 95% CI, 0.71–1.09; I2 = 25%), adverse events (OR, 0.98; 95% CI, 0.87–1.09; I2 = 17%), or mortality (OR, 0.96; 95% CI, 0.68–1.35; I2 = 7%). Patients
treated with imipenem were more likely to experience clinical or microbiological failure than those treated with alternative β-lactam monotherapy or combination therapy.
Conclusions
No differences in clinical outcomes were observed between carbapenems and noncarbapenem β-lactams in cIAIs. Patients treated with imipenem were more likely to experience clinical or microbiological failure than those treated with alternative β-lactam monotherapy or combination therapy.
Keywords: carbapenem, β-lactam, complicated intra-abdominal infections, meta-analysis, systematic review
Complicated intra-abdominal infections (cIAIs) are common in clinical practice and a frequent reason for hospitalization. They are often caused by a mixture of aerobic and anaerobic bacteria and have a high likelihood of developing into sepsis or septic shock [1]. Intra-abdominal infections consist of a wide spectrum of diseases, ranging from simple peritonitis or acute appendicitis to feculent diverticulitis or perforated appendicitis and other penetrating intra-abdominal injuries [2]. Common microorganisms isolated from intra-abdominal samples are gram-negative pathogens, mainly associated with normal enteric flora such as Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa [2, 3]. Patients with hospital-acquired infections have a higher prevalence of multidrug-resistant strains such as extended-spectrum β-lactam–producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and glycopeptide-resistant Enterococcus spp. [4–6].
The outcome of cIAIs, which are intra-abdominal infections extending beyond the wall of a hollow organ, depends on timely diagnosis and treatment including operative interventions and appropriate antimicrobial therapy [7]. The timing of antibiotic treatment also affects mortality, especially delays in antibiotic administration upon arrival at the hospital [8–10]. The antimicrobial therapies recommended for cIAIs in the guidelines by the Expert Panel of the Surgical Infection Society and the Infectious Diseases Society of America include carbapenem or other β-lactams as monotherapy or combination therapy [3].
Carbapenems have been proven to have a wider spectrum against bacteria in comparison with β-lactam antimicrobials, and therefore are often reserved for severe, complicated, and multidrug-resistant infections [11], and carbapenems also have a low propensity for the selection of mutants that are highly resistant to broad-spectrum cephalosporins [12]. However, resistance to carbapenems has emerged in Enterobacteriaceae, P. aeruginosa, and Acinetobacter baumannii [13]. Mortality attributable to carbapenem-resistant Enterobacteriaceae infections is 20%–54.3% and reflects the need for better treatment options [14].
Several meta-analyses were conducted to compare carbapenems with other β-lactams in common infections such as bacteremia and sepsis; they showed that β-lactam/β-lactamase inhibitors may be promising alternative antibiotics for definitive therapy in patients with bacteremia [15] and sepsis [16]. With the extensive clinical use of carbapenems and β-lactam monotherapy or combination therapy in cIAIs, more randomized controlled trials (RCTs) on this topic have been published; systematic reviews and meta-analyses of these antibiotic therapies, however, are scarce. Although carbapenems have documented efficacy in the treatment of cIAIs, it is unclear if this effect is consistent across the range of published studies. In times of rising antibiotic resistance and few new antibiotic agents in development, it is paramount that we understand the efficacy of individual drug classes. Due to the current lack of new antibiotic agents without overlapping mechanisms of resistance, judicious use of these broad-spectrum agents for treatment of resistant gram-negative infections is critical to preserve their future utility. We aimed to do a systematic review and meta-analysis of randomized controlled trials that compared the efficacy and safety of outcomes between carbapenem and alternative β-lactam treatments for cIAIs. For this purpose, we assessed clinical and microbiological treatment success as the primary end point. Adverse events and mortality were also assessed as the secondary outcomes.
METHODS
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.
Search Strategy and Selection Criteria
PubMed, Embase, Medline (via Ovid SP), and Cochrane library were systematically searched for all publications from inception through November 2018. The following search terms were used: “complicated intra-abdominal infections,” “cIAIs,” “carbapenem,” “imipenem,” “meropenem,” “biapenem,” “ertapenem,” “doripenem,” “faropenem,” “panipenem,” “razupenem,” “tebipenem,” “tomopenem,” and “sanfetrinem.” No language restriction was imposed. We included articles regardless of the language of publication and conference abstracts. The reference lists of all retrieved articles were also reviewed to identify additional articles missed by using these search terms. The authors approved all enrollment studies.
Inclusion Criteria
Studies meeting the following criteria were included: (i) population: patients with cIAIs; (ii) intervention: cIAI patients treated with carbapenems; (iii) comparison: cIAI patients treated with β-lactam monotherapy or combination therapy; (iv) outcome: primary outcomes: clinical treatment success and microbiological treatment success; secondary outcomes: all adverse events, mortality; (vi) design: randomized controlled trials (RCTs).
Exclusion Criteria
The exclusion criteria were (i) reviews, nonclinical studies, and case observations; (ii) not RCTs; (iii) reduplicated studies; (iv) improper outcome measures; (v) meta-analysees, case reports, and editorials.
Selection of Studies and Data Extraction
Comprehensive search of databases was performed by 2 researchers (Li and Chen), who deleted duplicate records, screened the titles and abstracts for relevance, and identified each article as excluded or requiring further assessment. We reviewed the full-text articles designated for inclusion and manually checked the references of the retrieved articles and previous reviews to identify additional eligible studies. Discrepancies were resolved by consensus. The following data were extracted from each study: study design, first author, year of publication, number of patients, age category (adult or child), interventions, comparisons, and outcomes.
Risk of Bias Assessment
Three reviewers (Li, Chen, and Jiang) independently evaluated the methodological quality of identified studies. The Risk of Bias Tool from the Cochrane Handbook for Systematic Reviews of Interventions, version 5.3.0, was used to assess methodological quality [17, 18]. In terms of the assessment criteria, each trial was rated and assigned to 1 of the 3 following levels of risk of bias: low: if all quality criteria were adequately met, the trial was deemed to have a low risk of bias; unclear: if 1 or more of the quality criteria was only partially met or was unclear, the trial was deemed to have a moderate risk of bias; or high: if 1 or more of the criteria were not met, or not included, the trial was deemed to have a high risk of bias [18, 19].
Data Analysis
Data were analyzed using the Review Manager 5.3.0. statistical package (Cochrane Collaboration Software). Dichotomous outcomes were expressed as odds ratios (ORs) with 95% confidence intervals (CIs). A test of heterogeneity was conducted with the I2 test and Q statistic, which is distributed as a χ 2 variate under the assumption of homogeneity of effect sizes. An I2 value >50% or P value <.05 was assumed to indicate significant heterogeneity [20]. Publication/reporting biases were visually assessed using funnel plots. If there was no observed heterogeneity, the fixed-effect model was chosen; otherwise a random-effects model was used [21].
Sensitivity analysis used a separate evaluation of studies with low risk of selection bias for concealment or double-blind design studies. Subgroup analyses were performed for the primary outcome, and based on the following: (i) stratifying the studies based on specific carbapenems such as ertapenem, imipenem/cilastatin, or meropenem vs β-lactam monotherapy or combination therapy; (ii) a subgroup of trials published since 2000 to reflect modern medicine; (iii) based on a clinical modified intent-to-treat (c-mITT) population or a microbiological modified intent-to-treat (m-mITT) population; (iv) based on specific antimicrobials, source of infection, and isolated pathogens.
RESULTS
Study Identification and Selection
A total of 1299 records were retrieved from the initial database search. After removing 390 duplicate articles, 909 records were eligible. Based on the inclusion and exclusion criteria, 790 articles were excluded after a simple reading of the titles and abstracts of the articles. The remaining 119 full-text articles were assessed for eligibility. Furthermore, studies with a design that was not relevant, non-RCTs, meta-analyses, studies with no outcomes, and those with mixed infections reported were excluded. Finally, a total of 22 RCT studies [22–43] were included in the meta-analysis. The selection process was conducted according to the PRISMA guidelines and is depicted in Figure 1. Among the 22 RCTs, 14 studies evaluated carbapenems compared with β-lactam combination therapy [22, 23, 25, 26, 29–34, 36, 38, 40, 42], β-lactam in combination with metronidazole was evaluated in 13 studies, and 1 study evaluated aztreonam combined with lindamycin [26]. Eight studies evaluated carbapenems compared with β-lactam monotherapy [24, 25, 27, 28, 35, 37, 41, 43], 7 studies evaluated piperacillin/tazobactam, and 1 trial evaluated ticarcillin/clavulanate [43]. As for carbapenems, ertapenem, imipenem/cilastatin, and meropenem were evaluated in 7, 8, and 7 studies, respectively.
Figure 1.
Selection process for the studies included in the meta-analysis.
Study Characteristics
The basic characteristics of the included studies are listed in Table 1, and the definition of clinical/microbiological and timing of evaluation are shown in Supplementary Table 1. Twenty-two RCTs studies involving 7720 participants were included in the analysis. These studies were published from 1987 to 2017. The number of participants in the studies ranged from 56 to 1066.
Table 1.
The Characteristics of the Included Studies
| Author/Year | Age Categories | Study Design | TOC, d | Site of Infection | APACHE II Scores | Treatment | No. |
|---|---|---|---|---|---|---|---|
| Angeras 1996 | A | Open RCT | 30 | Appendicitis, diverticulitis, biliary, or colon | ≤10: 83.72% | Imipenem/cilastatin 1.5–2.0 g/d | 515 |
| ≤10: 82.49% | Cefuroxime 3.0–4.5 g/d+ metronidazole 1.0–1.5 g/d | ||||||
| Barie 1997 | A | Double-blind RCT | 28–42 | cIAI, abscess, or peritonitis | Mean ± SD: 9.3 ± 8 | Imipenem/cilastatin 0.5 g × 4 | 350 |
| Mean ± SD: 7.8 ± 7 | Cefepime 2 g × 2 + metronidazole 500 mg (or 7.5 mg/kg) × 4 | ||||||
| Brismar 1992 | A | Open RCT | 7–14 and 28–42 | Appendicitis, peritonitis, pancreatitis, or salpingitis | NR | Imipenem/cilastatin 0.5 g/0.5 g × 3 | 134 |
| Piperacillin/tazobactam 4 g/0.5 g × 3 | |||||||
| Broze 1987 | A? | Open RCT | NA | Appendicitis, cholecystitis, colon, Crohn's disease, biliary ileus obstruction, peritonitis, perineal abscess, perforated disease | NR | Imipenem/cilastatin 0.5 g/0.5 g × 4 | 56 |
| Ceftazidime 4 g × 1 or ceftazidime 4 g × 1 + metronidazole 0.5 g × 3 | |||||||
| de Groot 1993 | B | Open RCT | NA | Stomach, gall bladder, small intestine, colon/rectum, or appendix | NR | Imipenem-cilastatin 0.5 g/0.5 g × 4 | 104 |
| Aztreonam 1 g × 3 + clindamycin 0.6 g × 3 | |||||||
| Dela Pena 2006 | A | Open RCT | 14 | Appendix, colon, gallbladder or biliary tract, stomach or duodenum, small intestine | ≤10: 96.1% | Ertapenem 1 g × 1 | 370 |
| ≤10: 95.8% | Piperacillin/tazobactam 3.375 g × 4 or 4.5 g × 3 | ||||||
| Erasmo 2004 | A | Open RCT | 28 ± 7 | Appendicitis, peritonitis, cholecystitis, cholangitis, or intra-abdominal abscess | NR | Imipenem/cilastatin 1 g × 4 | 293 |
| Piperacillin/tazobactam 4 g/0.5 g × 3 | |||||||
| Garbino 2007 | A | Double-blind RCT | 15–30 | Diffuse peritonitis, diverticulitis, appendicitis, perforated ulcer, or cholecystitis | Mean ± SD: 6.15 ± 4.13 | Imipenem/cilastatin 0.5 g/0.5 g × 4 | 122 |
| Mean ± SD: 5.48 ± 2.9 | Cefepime 2 g × 2 + metronidazole 0.5 g × 3 | ||||||
| Huizinga 1995 | A | Open RCT | 14–28 | Stomach, biliary/liver, pancreas, appendix, small bowel, or colon/rectum | ≤10: 81% | Meropenem 1 g × 3 | 160 |
| ≤10: 88% | Cefotaxime 2 g × 3 + metronidazole 0.5 g × 3 | ||||||
| Kempf 1996 | A | Open RCT | 14–28 | Perforated, peritonitis, appendix, colorectal, dehiscence, diverticulitis, and small bowel | ≤10: 60% | Meropenem 1 g × 3 | 83 |
| ≤10: 70% | Cefotaxime 2 g × 3 + metronidazole 0.5 g × 3 | ||||||
| Lucasti 2013 | A | Double-blind RCT | 14 | Appendix, stomach/duodenum, colon, small bowel, gall bladder, or parenchymal (liver or spleen) | ≤10: 83.3% | Meropenem 1 g × 3 | 204 |
| ≤10: 83.2% | Ceftazidime/avibactam 2 g/0.5 g × 3 + metronidazole 0.5 g × 3 | ||||||
| Lucasti 2014 | A | Double-blind RCT | 7–14 | Appendix, biliary, colon, stomach/duodenum, small bowel, liver, spleen, or biliary | ≤10: 85.7% | Meropenem 1 g × 3 | 122 |
| ≤10: 75% | Ceftolozane/tazobactam 1.5 g × 3 + metronidazole 0.5 g × 3 | ||||||
| Mazuski 2016 | A | Double-blind RCT | 28–35 | Cholecystitis, diverticular disease, appendiceal perforation or periappendiceal abscess, or secondary peritonitis | ≤10: 83.0% | Meropenem 1 g × 3 | 1066 |
| ≤10: 84.0% | Ceftazidime/avibactam 2 g/0.5 g × 3 + metronidazole 0.5 g × 3 | ||||||
| Namias 2007 | A | Double-blind RCT | 14 | Appendix, biliary-cholecystitis, colon, parenchymal (liver), small bowel | ≤10: 83.7% | Ertapenem 1 g × 1 | 535 |
| ≤10: 83.3% | Piperacillin/tazobactam 3.375 g × 4 | ||||||
| Narcisco 2005 | A | Double-blind RCT | 14 | Appendix, colon, gall bladder or biliary tract, stomach or duodenum, small intestine (beyond the duodenum) | ≤10: 97.3% | Ertapenem 1 g × 1 | 450 |
| ≤10: 95.6% | Ceftriaxone 2 g/d + metronidazole 30 mg/kg/d | ||||||
| Nord 1994 | A | Open RCT | 1–14 or 28–42 | NR | NR | Imipenem/cilastatin 1 g × 3 | 134 |
| Piperacillin/tazobactam 4.5 g× 3 | |||||||
| Qin 2017 | A | Double-blind RCT | 28–35 | Appendix, peritonitis, cholecystitis, intra-abdominal abscess, gastric and duodenal perforations, traumatic perforations, or diverticular disease | ≤10: 92.6% | Meropenem 1 g × 3 | 441 |
| ≤10: 93.9% | Ceftazidime/avibactam 2000/500 mg × 3 + metronidazole 500 mg × 3 | ||||||
| Solomkin 2003 | A | Double-blind RCT | 28–42 | Appendix, colon, gangrenous or abscessed, cholecystitis, small bowel, stomach/duodenum, or visceral abscess | ≤10: 70.44% | Ertapenem 1 g × 1 | 633 |
| ≤10: 75.13% | Piperacillin/tazobactam 3.375 g × 4 | ||||||
| Solomkin 2015 | A | Double-blind RCT | 24–32 | Appendix, biliary-cholecystitis, colon, stomach/duodenum, small bowel, parenchymal (liver or spleen), biliary cholangitis | Mean ± SD: 6.0 ± 4.1 | Meropenem 1 g × 3 | 993 |
| Mean ± SD: 6.2 ± 4.2 | Ceftolozane/tazobactam 1.5 g × 3 + metronidazole 500 mg × 3 | ||||||
| Teppler 2004 | A | Double-blind RCT | 28–42 | Appendicitis, colon, stomach/duodenum, small intestine, gall bladder/biliary tract, liver, spleen, subphrenic, subhepatic, or retroperitoneal abscess, uterus, pelvic inflammatory disease | NR | Ertapenem 1 g × 1 | 623 |
| Piperacillin/tazobactam 3.375 g × 4 | |||||||
| Yellin 2002 | A | Double-blind RCT | 28–42 | Appendix, colon, and another site | ≤10: 70.9% | Ertapenem 1 g × 1 or 1.5 g × 1 | 220 |
| ≤10: 73.6% | Ceftriaxone 2 g × 1 + metronidazole 0.5 g × 3 | ||||||
| Yellin 2007 | C | Open RCT | 14–35 | Appendicitis, gastrointestinal, peritonitis, or pelvic abscess | NR | Ertapenem 15 mg/kg × 2 or 1 g × 1 | 112 |
| Ticarcillin/clavulanate 50 mg/kg or ticarcillin 3 g and clavulanic acid 0.1 g |
Abbreviations: A, adult; APACHE, Acute Physiology and Chronic Health Evaluation; B, both adult and child; C, child; cIAI, complicated intra-abdominal infection; NR, not reported; RCT, randomized controlled trial; TOC, test of cure.
Risk of Bias Assessment
The Cochrane Risk of Bias Tool was used to assess the quality of our study [17]. The outcomes of the risk of bias are summarized in Supplementary Figure 1A and Supplementary Figure 1B. Almost half of the included RCTs were assessed to be of a high methodological quality. Twelve studies [23, 29, 32–36, 38–42] had low risk for sequence generation and allocation concealment. Nine trials [22, 24, 25, 27, 28, 30, 31, 37, 43] were performed using an open-label model, with a high risk for performance bias and detection bias, and 1 RCT [26] did not mention whether blinding procedures was used.
As for attrition bias, all trials had a low risk of bias for selective outcome reporting, and all but 2 had a low risk of bias for incomplete outcome data reporting [22, 30].
In addition to other biases, 2 studies, Huizinga et al. [30] and Kempf et al. [31], were reported to have an unbalanced Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II score, with higher scores in the carbapenems group.
Clinical Treatment Success
Twenty-one studies including a total of 5088 patients provided data on clinical treatment success. Compared with carbapenems, β-lactams showed no significant difference in terms of clinical success (OR, 0.86; 95% CI, 0.71–1.05; P = .13; I2 = 35%) (Figure 2). Furthermore, in subgroup analysis by type of carbapenem compared with β-lactam monotherapy or combination therapy, we found that patients treated with imipenem/cilastain were more likely to experience clinical failure vs alternative β-lactam, regardless of whether it was monotherapy or combination therapy (OR, 0.38; 95% CI, 0.21–0.67; P < .01; I2 = 46%; OR, 0.66; 95% CI, 0.46–0.96; P = .03; I2 = 26%, respectively) (Figure 2). No clinical treatment success difference was found between ertapenem with β-lactam monotherapy or combination therapy (OR, 0.99; 95% CI, 0.58–1.68; P = .96; I2 = 0%; OR, 1.07; 95% CI, 0.53–2.18; P = .85; I2 = 0%, respectively) (Figure 2). Similar results were observed in meropenem vs β-lactam combination therapy (OR, 1.25; 95% CI, 0.91–1.71; P = .17; I2 = 0%) (Figure 2).
Figure 2.

Forest plot of clinical treatment success. Abbreviation: CI, confidence interval.
In a subgroup analysis including studies since 2000, there was no significant difference between carbapenems and alternative β-lactam (OR, 1.01; 95% CI, 0.79–1.29; P = .94; I2 = 0%) (Supplementary Figure 2). Similar results were observed in a subgroup analysis including studies only for a c-mITT population (OR, 1.11; 95% CI, 0.95–1.29; P = .19; I2 = 34%) (Supplementary Figure 3).
A sensitivity analysis using trials with low risk of selection bias due to concealment is presented in Supplementary Figure 4; it showed similar results (OR, 0.92; 95% CI, 0.71–1.18; P = .50; I2 = 0%). Similar results of trials with a double-blind design are shown in Supplementary Figure 5 (OR, 0.90; 95% CI, 0.70–1.15; P = .38; I2 = 0%).
Microbiological Treatment Success
Microbiological treatment success was evaluated by 3708 investigators in the 19 trials, although explicit definitions of microbiological treatment success were only reported for some trials (Supplementary Table 1). Microbiological treatment success occurred in 1717/1936 patients (88.69%) treated with a carbapenem and 1596/1772 (90.07%) treated with an alternative β-lactam monotherapy or combination therapy. There were no significant differences in microbiological treatment success between carbapenems and alternative β-lactam monotherapy or combination therapy (OR, 0.88; 95% CI, 0.71–1.09; P = .24; I2 = 25%) (Figure 3). In a subgroup analysis by type of carbapenem compared with β-lactam monotherapy or combination therapy, similar results were shown, that patients treated with imipenem/cilastain were more likely to experience microbiological failure vs those treated with alternative β-lactam, regardless of whether it was monotherapy or combination therapy (OR, 0.33; 95% CI, 0.15–0.73; P = .006; I2 = 0%; OR, 0.55; 95% CI, 0.35–0.85; P = .007; I2 = 0%, respectively) (Figure 3). No microbiological treatment success difference was found between ertapenem with β-lactam monotherapy vs combination therapy (OR, 1.12; 95% CI, 0.79–1.58; P = .54; I2 = 28%; OR, 1.51; 95% CI, 0.64–3.59; P = .35; I2 = 0%, respectively) (Figure 3). Similar results were observed in meropenem vs β-lactam combination therapy (OR, 1.28; 95% CI, 0.79–2.08; P = .31; I2 = 0%) (Figure 3).
Figure 3.
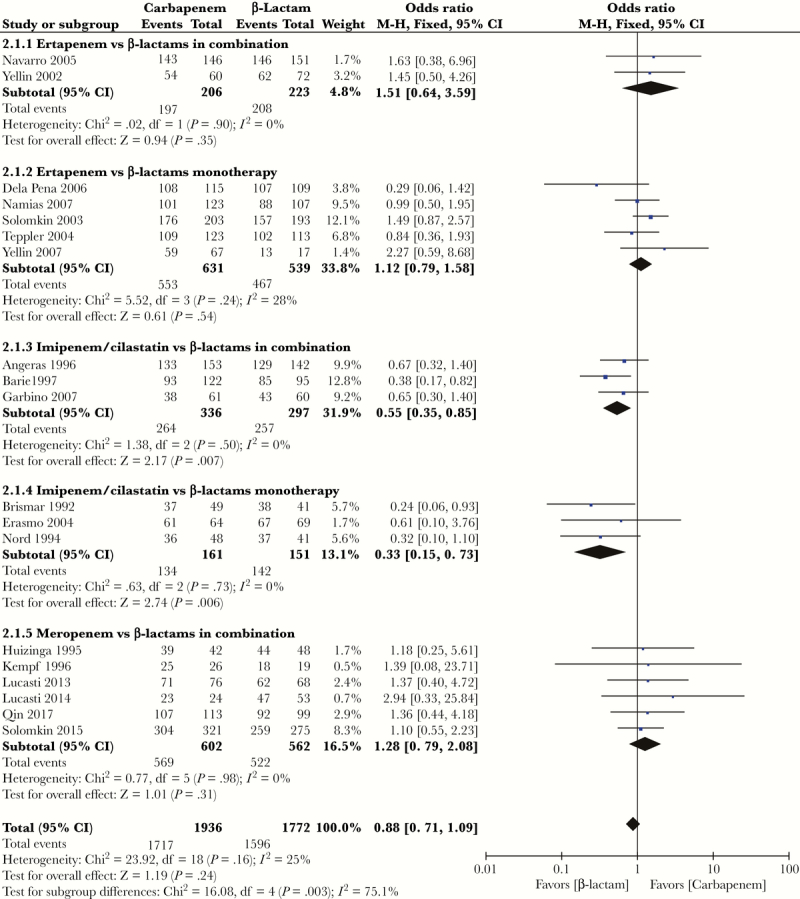
Forest plot of microbiological treatment success. Abbreviation: CI, confidence interval.
In a subgroup analysis including studies since 2000, there was no significant difference between carbapenems and alternative β-lactam (OR, 1.10; 95% CI, 0.86–1.42; P = .44; I2 = 0%) (Supplementary Figure 6). However, in a subgroup analysis including an m-mITT population, patients treated with carbapenems were more likely to experience microbiological treatment success vs alternative β-lactam monotherapy or combination therapy (OR, 1.50; 95% CI, 1.20–1.87; P = .0003; I2 = 0%) (Figure 4).
Figure 4.
Forest plots of subgroup analysis of microbiological treatment success including only studies of microbiological modified intent-to-treat populations. Abbreviation: CI, confidence interval.
In a subgroup analysis including patients with infection caused by E. coli, K. pneumoniae, P. aeruginosa, Enterobacter spp., and Enterococcus spp., no significant difference was observed between carbapenems and alternative β-lactam monotherapy or combination therapy (Table 2; Supplementary Figure 7).
Table 2.
Subgroup Analysis of Per-Pathogen Microbiological Treatment Success
| No. of Patients Successfully Treated/Total No. (%) | OR (95% CI) | ||
|---|---|---|---|
| Carbapenems | β-Lactams | ||
| E. coli | 814/929 (87.62) | 866/968 (89.37) | 0.84 (0.63–1.12) |
| K. pneumoniae | 128/147 (87.07) | 112/129 (86.82) | 1.05 (0.52–2.15) |
| P. aeruginosa | 105/114 (92.11) | 87/97 (89.69) | 1.24 (0.51–3.02) |
| Enterobacter spp. | 127/134 (94.78) | 96/103 (93.20) | 1.69 (0.59–4.85) |
| Enterococcus spp. | 60/72 (83.33) | 55/61 (90.16) | 0.65 (0.23–1.82) |
Abbreviations: CI, confidence interval; OR, odds ratio.
A sensitivity analysis using trials with low risk of selection bias due to concealment is presented in Supplementary Figure 8; it showed similar results (OR, 0.96; 95% CI, 0.73–1.25; P = .74; I2 = 34%). Similar results of trials with a double-blind design are shown in Supplementary Figure 9 (OR, 1.00; 95% CI, 0.78–1.29; P = .98; I2 = 16%).
Adverse Events
Twenty studies included a total of 6812 patients who had experienced adverse events (AEs). This analysis revealed no significant difference between carbapenems and alternative β-lactam monotherapy or combination therapy (OR, 0.98; 95% CI, 0.87–1.09; P = .66; I2 = 17%) (Supplementary Figure 10). Similar results were observed in analyses of patients who had experienced serious clinical AEs (OR, 1.09; 95% CI, 0.87–1.37; P = .44; I2 = 0%) (Supplementary Figure 11) and AEs leading to discontinuation (OR, 1.04; 95% CI, 0.70–1.52; P = .86; I2 = 0%) (Supplementary Figure 12).
Mortality
Mortality was reported in 14 trials. No mortality differences were noted between those treated with carbapenems vs those treated with alternative β-lactam monotherapy or combination therapy according to a meta-analysis (OR, 0.96; 95% CI, 0.68–1.35; P = .81; I2 = 7%) (Supplementary Figure 13).
Discussion
In the present study, we systematically reviewed all published RCTs comparing carbapenem-based regimens vs alternative β-lactam-based regimens of monotherapy or combination therapy for the treatment of patients with cIAIs. The results of the primary outcomes showed no difference in the clinical and microbiological treatment success or in the risk of AEs and mortality among cIAI patients treated with either a carbapenem or a noncarbapenem β-lactam agent monotherapy or combination therapy. In general, subgroup analyses of cIAIs did not reveal an advantage for using carbapenems for either of the subgroups tested, including type of carbapenem, year of publication, modified ITT population, and type of pathogen. Exceptions were microbiological treatment success in the subpopulation of ITT, which was significantly more likely with carbapenems. In addition, for the outcomes of clinical and microbiological success, significantly lower success rates were noted with imipenem as the comparator for carbapenem.
Few meta-analysis studies have compared the efficacy and safety of combined therapy with carbapenems vs alternative β-lactam single or combination therapy for the treatment of cIAIs in recent years. A similar meta-analysis conducted in 2016 investigated the efficacy of metronidazole combination therapies and carbapenem and suggested that combined therapy with metronidazole can be an effective and safe treatment option for cIAI, similar to carbapenems [44]. However, this study did not include β-lactam monotherapy and included only 8 trials comparing β-lactams vs carbapenems.
Antimicrobial therapy for cIAIs is prescribed empirically. The Surgical Infection Society, the Infectious Diseases Society of America, and the World Society of Emergency Surgery published guidelines on this issue, suggesting that fluroquinolones and β-lactams alone or, when needed, in combination with metronidazole are equally effective [45]. The choice of therapy is physician-oriented and is based on the local bacterial epidemiology, the infection site, acquisition (eg, community-acquired, health care–associated, or hospital-acquired), and risk for treatment failure and death [46].
In 2014, the intravenous formulation of metronidazole was approved in Japan, long after the oral formulation was first approved in 1961 [44]. When evaluating carbapenems compared with β-lactam combination therapy, our study showed results similar to those published in previous study, and the findings of this analysis confirm the majority of the recommendations regarding antibiotic treatment for patients with cIAIs. On the other hand, our analysis also focused on RCTs, which is the only design that allows the testing of interventions with β-lactam monotherapy for patients with cIAIs. Similar results were observed with carbapenems compared with β-lactam monotherapy. Piperacillin/tazobactam is one of the currently recommended agents for empiric treatment of cIAI in lower-risk patients, and the current recommendation is to reserve this agent for higher-risk patients because of its broader-spectrum antimicrobial activity [45].
An increased risk of failure of clinical and microbiological treatment has been observed in patients treated with imipenem/cilastatin compared with β-lactam monotherapy or combination therapy. Most of the included studies that evaluated imipenem/cilastatin were published before 2000 and might not reflect modern medicine. These features are increasingly crucial to guide therapy and outline optimal evidence-based management recommendations. On the other hand, ertapenem and meropenem showed similar clinical and microbiological treatment success compared with β-lactam monotherapy or combination therapy. However, carbapenem-resistant Enterobacteriaceae including Klebsiella spp. and E. coli, in particular, has increased dramatically in the last decade. There are limited therapeutic options available for infections caused by carbapenem-resistant Enterobacteriaceae; therefore, these infections are difficult to treat and associated with high mortality rates [47], and there is a critical need to use existing agents carefully to prevent the emergence of drug-resistant bacteria.
This meta-analysis has certain limitations. First, the pharmacokinetic data of antibacterial agents used in the included studies were not reported, which restricts the evaluation of the adequacy of the administered doses, which might lead to a bias in the pooled effect. Second, intra-abdominal infections encompass a wide range of infections with different bacterial patterns that depend on the source, such as gastroduodenal vs appendix vs colon, and it is possible that certain antimicrobials perform better for upper or lower gastrointestinal infections and worse for other infections, but cumulatively they appear equal. In addition, variables including age, sex, underlying disease, nutritional status, and assessment time point of patients were also potential bias-inducing factors. Third, source control is of paramount importance in patients with cIAIs and is difficult to standardize [48]; This study used the primary outcome of clinical and microbiological treatment success to reduce the likelihood of misclassification bias, but the identified studies used different definitions of “clinical and microbiological treatment success.” Fourth, the enrolled patients were not stratified by severity of illness, and mortality was 2%, which suggests that the most severely ill patients were under-represented in the analyzed trials [49] and extrapolation of our findings in this population should be performed with caution. Last but not least, about 40% of included trials were open label, which may lead to increased risk of performance and detection bias, especially in subjective outcomes.
Conclusions
In conclusion, the current meta-analysis revealed that β-lactam monotherapy or combination therapy can be an effective and safe treatment option for cIAI, similar to carbapenem. Ertapenem and meropenem appear to be similarly effective to other β-lactam monotherapy or combination therapy for a variety of pathogens that cause cIAIs. However, increased risk of failure of clinical and microbiological treatment was observed in patients treated with imipenem/cilastatin compared with β-lactam monotherapy or combination therapy. The results were consistent when differences in therapy drug type and potential cofounders of the identified studies were considered. In addition, empiric antimicrobial treatment of patients with cIAIs should be selected in light of the local bacterial epidemiology and patterns of resistance. Further research may focus on subgroups such as patients with health care–associated cIAIs and critically ill patients; clinical outcomes should ideally be stratified by infection site, such as appendicitis.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This project was supported by the scientific research and technological development projects of Hechi, Guangxi Province of China (Heke B1824-4).
Potential conflicts of interest. The authors have indicated that they have no conflicts of interest regarding the content of this article. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Eckmann C, Dryden M, Montravers P, et al. Antimicrobial treatment of “complicated” intra-abdominal infections and the new IDSA guidelines? A commentary and an alternative European approach according to clinical definitions. Eur J Med Res 2011; 16:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berlin A, Johanning JM. Intraabdominal infections in older adults. Clin Geriatr Med 2016; 32:493–507. [DOI] [PubMed] [Google Scholar]
- 3. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:133–64. [DOI] [PubMed] [Google Scholar]
- 4. Montravers P, Lepape A, Dubreuil L, et al. Clinical and microbiological profiles of community-acquired and nosocomial intra-abdominal infections: results of the French prospective, observational EBIIA study. J Antimicrob Chemother 2009; 63:785–94. [DOI] [PubMed] [Google Scholar]
- 5. Sartelli M, Catena F, Ansaloni L, et al. Complicated intra-abdominal infections in Europe: a comprehensive review of the CIAO study. World J Emerg Surg 2012; 7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee YL, Chen YS, Toh HS, et al. Antimicrobial susceptibility of pathogens isolated from patients with complicated intra-abdominal infections at five medical centers in Taiwan that continuously participated in the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2006 to 2010. Int J Antimicrob Agents 2012; 40:S29–36. [DOI] [PubMed] [Google Scholar]
- 7. Falagas ME, Peppas G, Makris GC, et al. Meta-analysis: ertapenem for complicated intra-abdominal infections. Aliment Pharmacol Ther 2008; 27:919–31. [DOI] [PubMed] [Google Scholar]
- 8. Sartelli M. A focus on intra-abdominal infections. World J Emerg Surg 2010; 5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montravers P, Tashk P, Tran Dinh A. Unmet needs in the management of intra-abdominal infections. Expert Rev Anti Infect Ther 2017; 15:839–50. [DOI] [PubMed] [Google Scholar]
- 10. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–96. [DOI] [PubMed] [Google Scholar]
- 11. Zhanel GG, Wiebe R, Dilay L, et al. Comparative review of the carbapenems. Drugs 2007; 67:1027–52. [DOI] [PubMed] [Google Scholar]
- 12. Goldstein FW. Cephalosporinase induction and cephalosporin resistance: a longstanding misinterpretation. Clin Microbiol Infect 2002; 8:823–5. [DOI] [PubMed] [Google Scholar]
- 13. Zhanel GG, Lawrence CK, Adam H, et al. Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem-β-lactamase inhibitor combinations. Drugs 2018; 78:65–98. [DOI] [PubMed] [Google Scholar]
- 14. Kaye KS, Bhowmick T, Metallidis S, et al. Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the TANGO I randomized clinical trial. JAMA 2018; 319:788–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Son SK, Lee NR, Ko JH, et al. Clinical effectiveness of carbapenems versus alternative antibiotics for treating ESBL-producing Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother 2018; 73:2631–42. [DOI] [PubMed] [Google Scholar]
- 16. Shiber S, Yahav D, Avni T, et al. β-lactam/β-lactamase inhibitors versus carbapenems for the treatment of sepsis: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 2015; 70:41–7. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Green S. Cochrane Reviewers’ Handbook 5.3.0. Version 5.3.0. The Cochrane Collaboration. 2014. Available at: www.cochrane-handbook.org. Accessed 20 April 2019.
- 19. Shiber S, Yahav D, Avni T, et al. β-lactam/β-lactamase inhibitors versus carbapenems for the treatment of sepsis: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 2015; 70:41–7. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Falagas ME, Peppas G, Makris GC, et al. Meta-analysis: ertapenem for complicated intra-abdominal infections. Aliment Pharmacol Ther 2008; 27:919–31. [DOI] [PubMed] [Google Scholar]
- 22. Angerås MH, Darle N, Hamnström K, et al. A comparison of imipenem/cilastatin with the combination of cefuroxime and metronidazole in the treatment of intra-abdominal infections. Scand J Infect Dis 1996; 28:513–8. [DOI] [PubMed] [Google Scholar]
- 23. Barie PS, Vogel SB, Dellinger EP, et al. A randomized, double-blind clinical trial comparing cefepime plus metronidazole with imipenem-cilastatin in the treatment of complicated intra-abdominal infections. Cefepime Intra-abdominal Infection Study Group. Arch Surg 1997; 132:1294–302. [DOI] [PubMed] [Google Scholar]
- 24. Brismar B, Malmborg AS, Tunevall G, et al. Piperacillin-tazobactam versus imipenem-cilastatin for treatment of intra-abdominal infections. Antimicrob Agents Chemother 1992; 36:2766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Broze B, De Mees J, Droissart R, et al. Randomized comparison of imipenem/cilastatin and ceftazidime in the empiric therapy of severe abdominal infections a multicenter study. Acta Clin Belg 1987; 42:431–6. [DOI] [PubMed] [Google Scholar]
- 26. de Groot HG, Hustinx PA, Lampe AS, Oosterwijk WM. Comparison of imipenem/cilastatin with the combination of aztreonam and clindamycin in the treatment of intra-abdominal infections. J Antimicrob Chemother 1993; 32:491–500. [DOI] [PubMed] [Google Scholar]
- 27. Dela Pena AS, Asperger W, Köckerling F, et al. ; Optimizing Intra-Abdominal Surgery with Invanz (OASIS)-I Study Group Efficacy and safety of ertapenem versus piperacillin-tazobactam for the treatment of intra-abdominal infections requiring surgical intervention. J Gastrointest Surg 2006; 10:567–74. [DOI] [PubMed] [Google Scholar]
- 28. Erasmo AA, Crisostomo AC, Yan LN, et al. Randomized comparison of piperacillin/tazobactam versus imipenem/cilastatin in the treatment of patients with intra-abdominal infection. Asian J Surg 2004; 27:227–35. [DOI] [PubMed] [Google Scholar]
- 29. Garbino J, Villiger P, Caviezel A, et al. A randomized prospective study of cefepime plus metronidazole with imipenem-cilastatin in the treatment of intra-abdominal infections. Infection 2007; 35:161–6. [DOI] [PubMed] [Google Scholar]
- 30. Huizinga WK, Warren BL, Baker LW, et al. Antibiotic monotherapy with meropenem in the surgical management of intra-abdominal infections. J Antimicrob Chemother 1995; 36(Suppl A):179–89. [DOI] [PubMed] [Google Scholar]
- 31. Kempf P, Bauernfeind A, Müller A, Blum J. Meropenem monotherapy versus cefotaxime plus metronidazole combination treatment for serious intra-abdominal infections. Infection 1996; 24:473–9. [DOI] [PubMed] [Google Scholar]
- 32. Lucasti C, Popescu I, Ramesh MK, et al. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind, phase II trial. J Antimicrob Chemother 2013; 68:1183–92. [DOI] [PubMed] [Google Scholar]
- 33. Lucasti C, Hershberger E, Miller B, et al. Multicenter, double-blind, randomized, phase II trial to assess the safety and efficacy of ceftolozane-tazobactam plus metronidazole compared with meropenem in adult patients with complicated intra-abdominal infections. Antimicrob Agents Chemother 2014; 58:5350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mazuski JE, Gasink LB, Armstrong J, et al. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis 2016; 62:1380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Namias N, Solomkin JS, Jensen EH, et al. Randomized, multicenter, double-blind study of efficacy, safety, and tolerability of intravenous ertapenem versus piperacillin/tazobactam in treatment of complicated intra-abdominal infections in hospitalized adults. Surg Infect (Larchmt) 2007; 8:15–28. [DOI] [PubMed] [Google Scholar]
- 36. Navarro NS Jr, Campos MI, Alvarado R, et al. ; Oasis II Study Team Ertapenem versus ceftriaxone and metronidazole as treatment for complicated intra-abdominal infections. Int J Surg 2005; 3:25–34. [DOI] [PubMed] [Google Scholar]
- 37. Nord CE. The treatment of severe intra-abdominal infections: the role of piperacillin/tazobactam. Intensive Care Med 1994; 20(Suppl 3):S35–8. [DOI] [PubMed] [Google Scholar]
- 38. Qin X, Tran BG, Kim MJ, et al. A randomised, double-blind, phase 3 study comparing the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem for complicated intra-abdominal infections in hospitalised adults in Asia. Int J Antimicrob Agents 2017; 49:579–88. [DOI] [PubMed] [Google Scholar]
- 39. Solomkin JS, Yellin AE, Rotstein OD, et al. Ertapenem versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections: results of a double-blind, randomized comparative phase III trial. Ann Surg 2003; 237:235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Solomkin J, Hershberger E, Miller B, et al. Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin Infect Dis 2015; 60:1462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Teppler H, Meibohm AR, Woods GL. Management of complicated appendicitis and comparison of outcome with other primary sites of intra-abdominal infection: results of a trial comparing ertapenem and piperacillin-tazobactam. J Chemother 2004; 16:62–9. [DOI] [PubMed] [Google Scholar]
- 42. Yellin AE, Hassett JM, Fernandez A, et al. ; 004 Intra-abdominal Infection Study Group Ertapenem monotherapy versus combination therapy with ceftriaxone plus metronidazole for treatment of complicated intra-abdominal infections in adults. Int J Antimicrob Agents 2002; 20:165–73. [DOI] [PubMed] [Google Scholar]
- 43. Yellin AE, Johnson J, Higareda I, et al. Ertapenem or ticarcillin/clavulanate for the treatment of intra-abdominal infections or acute pelvic infections in pediatric patients. Am J Surg 2007; 194:367–74. [DOI] [PubMed] [Google Scholar]
- 44. Mikamo H, Yuasa A, Wada K, et al. Optimal treatment for complicated intra-abdominal infections in the era of antibiotic resistance: a systematic review and meta-analysis of the efficacy and safety of combined therapy with metronidazole. Open Forum Infect Dis 2016; 3:ofw143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mazuski JE, Tessier JM, May AK, et al. The Surgical Infection Society revised guidelines on the management of intra-abdominal infection. Surg Infect 2017; 18:1–76. [DOI] [PubMed] [Google Scholar]
- 46. De Simone B, Coccolini F, Catena F, et al. Benefits of WSES guidelines application for the management of intra-abdominal infections. World J Emerg Surg 2015; 10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 2011; 53:60–7. [DOI] [PubMed] [Google Scholar]
- 48. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Solomkin JS, Ristagno RL, Das AF, et al. Source control review in clinical trials of anti-infective agents in complicated intra-abdominal infections. Clin Infect Dis 2013; 56:1765–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




