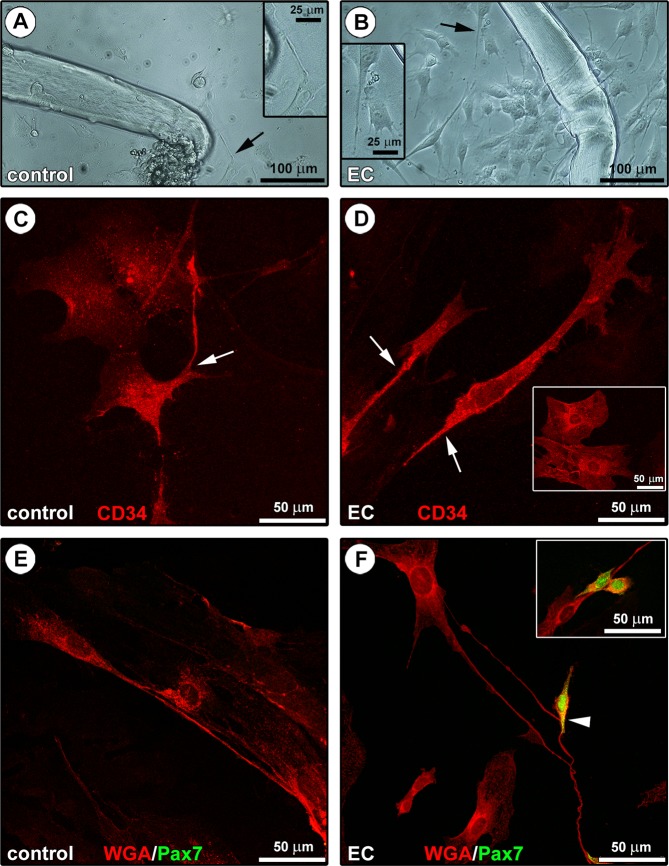
Figure 5.
Morphological evaluation of the in vitro interaction between telocytes (TCs) and satellite cells (SCs) from single living endomysial sheath-covered myofibers. (A,B) Phase-contrast microscopic images of endomysial sheath-covered myofibers from control and eccentric contraction (EC)-damaged muscles after 48 hours of culture on Matrigel-coated plates; sprouting cells appear more numerous around EC-injured myofibers. Note that some of these morphologically different cells display the typical features of TCs (i.e. moniliform/varicose telopodes; arrows and higher magnifications in the insets). (C,D) CD34 confocal immunofluorescence staining. Cells with a polygonal or spindle-shaped cell body abruptly giving rise to thin moniliform processes (arrows) and displaying a strong CD34 positivity, thus identifiable as TCs, are detected in the heterogeneous cell population derived from either control (C) or EC-injured (D) myofibers. These cells are distinguishable from typically fried egg-shaped CD34+ endothelial cells (D, inset). (E,F) WGA and Pax7 confocal double fluorescent staining. The presence of telopode-bearing TCs in control and EC-damaged myofiber-derived cells is confirmed by labeling with the membrane dye WGA. Pax7+ SCs, undetectable at this experimental time in the control myofiber-derived cell population (E), are instead detected in cell cultures from EC-damaged myofibers (F). Of note, SCs are often observed in cell pairs presumably resulting from mitotic division (F, inset) and in contact with TCs/telopodes (F, arrowhead and inset). Images are representative of at least 6 cell preparations from each of 3 control and 3 EC-injured muscle samples.