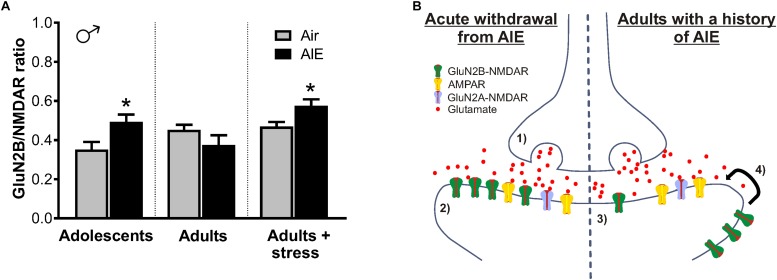
FIGURE 9.
Summary of AIE-induced changes in GluN2B-containing NMDARs of the male dlBNST and a proposed model of the synaptic mechanisms underlying these changes. (A) GluN2B inhibition (produced by Ro 25–6981) normalized to total NMDAR transmission. Symbols indicate significant differences between groups, as determined by an unpaired t-test, ∗p < 0.05. Under basal conditions, adults with a history of AIE exhibited reduced GluN2B (p = 0.1868)—a trend that was opposite to that seen immediately following AIE and in stressed adults with a history of AIE. (B) Proposed model of AIE-induced changes to synaptic GluN2B-containing NMDARs during acute and protracted withdrawal, as well as during adult stress. Acute withdrawal from AIE (1) enhances presynaptic glutamate release and (2) increases in the GluN2B-containing NMDARs at the postsynaptic density. The lateral movement of these GluN2B-NMDARs to extrasynaptic locations occurs during extended withdrawal from AIE (3). Stress during extended withdrawal triggers the retargeting of extrasynaptically located GluN2B-NMDARs to the synapse (4), revealing AIE-induced changes in GluN2B-NMDAR transmission and NMDAR metaplasticity.