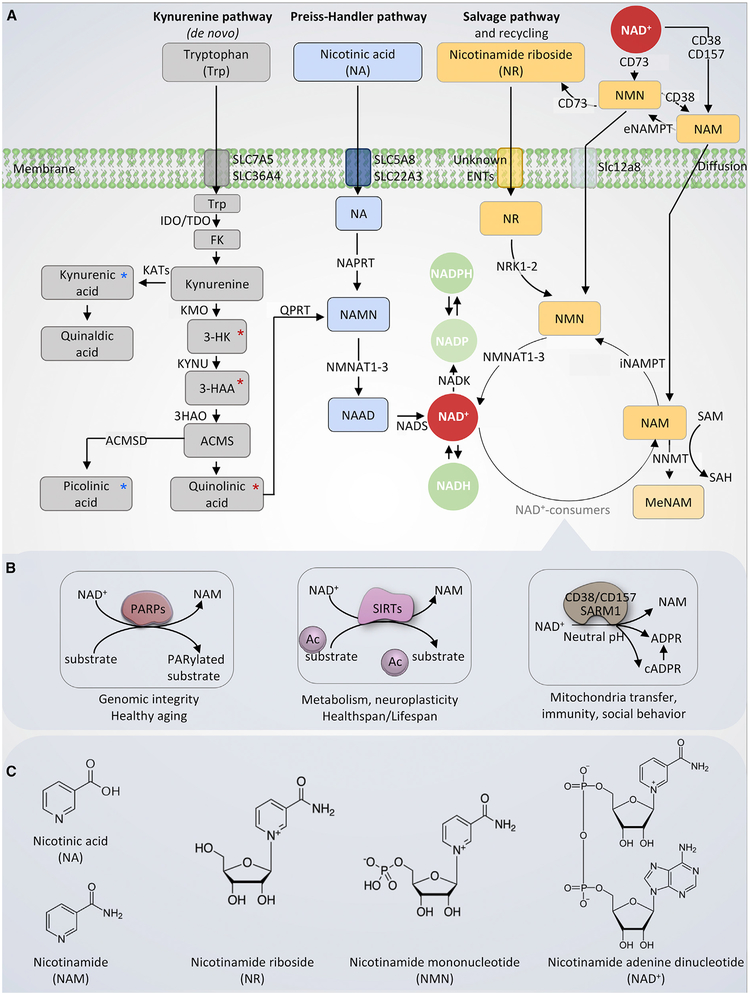
Figure 1. Nicotinamide Adenine Dinucleotide (NAD+) Production and Catabolism in Mammalian Cells.
(A) NAD+ is produced via three major pathways in mammals. The first is the de novo biosynthesis from tryptophan (Trp), also called the kynurenine pathway. Trp enters the cell via the transporters SLC7A5 and SLC36A4. Within the cell, Trp is converted to formylkynurenine (FK), which is further converted to kynurenine. Kynurenine can be converted to kynurenic acid (via kynurenine aminotransferases/KATs) and finally quinaldic acid. Additionally, kynurenine can be converted to 3-hydroxykynurenine (3-HK) by kynurenine 3-monooxygenase (KMO), and further to 3-hydroxyanthranilic acid (3-HAA) by tryptophan 2,3-dioxygenase (KYNU). The next step is performed by 3-hydroxyanthranilic acid oxygenase (3HAO) to produce α-amino-β-carboxymuconate-ϵ-semialdehyde (ACMS). Via a spontaneous reaction, ACMS converts to quinolinic acid, which further formulates to NAMN by quinolinate phosphoribosyltransferase (QPRT), and to nicotinic acid adenine dinucleotide (NAAD), and finally to NAD+. 3-HK, 3-HAA, and quinolinic acid are neurotoxic (denoted with red asterisk), whereas kynurenic acid and picolinic acid are neuroprotective (marked with green asterisk). The second pathway is the Preiss-Handler pathway during which nicotinic acid (NA) is used as an NAD+ precursor. NA enters the cell via SLC5A8 or SLC22A3 transporters. The Preiss-Handler pathway is initiated by the conversion of NA to NAMN by NA phosphoribosyl-transferase (NAPRT). NAMN, an intermediate in both kynurenine pathway and the Preiss-Handler pathway, is converted to form NAAD by NAM mononucleotide transferases (NMNATs). Lastly, NAAD is converted to NAD+ by NAD+ synthase (NADS). The third pathway is the salvage pathway with the cells generating NAD+ from nicotinamide riboside (NR) and recycling nicotinamide (NAM) back to NAD+ via nicotinamide mononucleotide (NMN). Extracellularly, NAD+ or NAM can be converted to NMN, which is in turn dephosphorylated to NR, possibly by CD73. NR is transported into the cell via an unknown nucleoside transport. Intracellularly, NR forms NMN via NRK1 or NRK2 in a tissue-specific manner. NMN is then converted to NAD+ by NMNATs. The enzyme NAM N-methyltransferase (NNMT) methylates NAM, using S-adenosyl methionine (SAM) as a methyl donor. This removes NAM from recycling and indirectly affects NAD+ levels.
(B) The four major NAD+-consuming enzymes. From left: poly(ADP-ribose) polymerases (PARPs), especially PARP1 and PARP2, use NAD+ as a co-substrate to PARylate target proteins, generating NAM as a by-product. The deacetylation activity of sirtuin (SIRT)1, SIRT3, and SIRT6 depends on NAD+, generating NAM as a by-product, with NAM at high cellular levels inhibiting the activity of SIRTs. The NADases or cyclic ADP-ribose synthases (cADPRSs) CD38 and CD157 hydrolyze NAD+ to NAM, generating ADPR and cADPR; in addition, CD38 can degrade NMN to NAM, removing NMN from NAD+ synthesis. Sterile alpha and TIR motif-containing 1 (SARM1) was recently identified as a NADase, which cleaves NAD+ to NAM, cADPR, and ADPR.
(C) The chemical structures of NAD+ and the NAD+ precursors. From left: nicotinic acid (NA), nicotinamide (NAM), nicotinamide riboside (NR), nicotinamide mononucleotide (NMN), and oxidized form of nicotinamide adenine dinucleotide (NAD+).
Note that CD38, CD73, and CD157 are membrane proteins. For simplicity, we did not attach them to the membrane in this schematic model.