FIGURE 1.
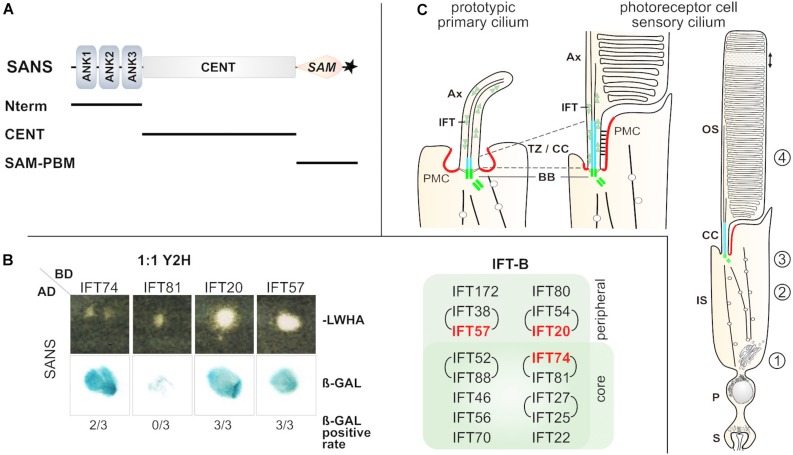
SANS-IFT binary interactions were identified via 1:1 Yeast-two-hybrid. (A) Scheme of domain composition of human SANS protein. SANS has 461 aa and a molecular weight of 51 kDa and is composed of the following domains: 3 ANK, ankyrin repeats in the SANS_Nterm; CENT, central domain; SAM, sterile alpha motif; ∗PBM, PDZ binding motif. SANS deletion constructs, which were used for GFP-Traps are indicated below by black lines. (B) 1:1 Y2H assay. Representative yeast colonies on –LWHA selection media and positive ß-galactosidase assay revealed binding of SANS full length to several IFT-B proteins. For all IFTs three Y2H assays were performed. The rate of ß-GAL positive tests is indicated below, e.g., 2 out of 3 for IFT74 (2/3). Positive hits are highlighted in the IFT-B composition overview in red. (C) Schemes of a prototypic cilium and a photoreceptor cilium. Both share homolog structures and common ciliary transport modules: the outer segment (OS) of photoreceptors resembles the ciliary shaft with axoneme (Ax) of the prototypic cilium, and the connecting cilium corresponds to the transition zone (TZ, blue). Intracellular transport for the delivery of ciliary proteins to the outer segment is organized in multiple modules: cargo sorting and vesicle budding at the Golgi apparatus (1), microtubule guided vectorial transport through the inner segment via cytoplasmic dynein (2), vesicle fusion with the periciliary membrane complex (PCM, red) and cargo reloading/assembly at the ciliary base (3) and finally, intra-ciliary transport along the CC/TZ and the Ax (4), mediated by intraflagellar transport (IFT) complexes (IFT-B/kinesin II or IFT-A/dynein-2) or by myosin VIIa, respectively. BB, basal body, green; P, perikaryon, S, synapse.