Keywords: nerve regeneration, schizophrenia, MK-801, N-methyl-D-aspartate, neurogenesis, N-methyl-D-aspartate receptor, N-methyl-D-aspartate receptor subunit 1, BrdU, Ki67, hippocampal dentate gyrus, hippocampal neurogenesis, neural regeneration
Abstract
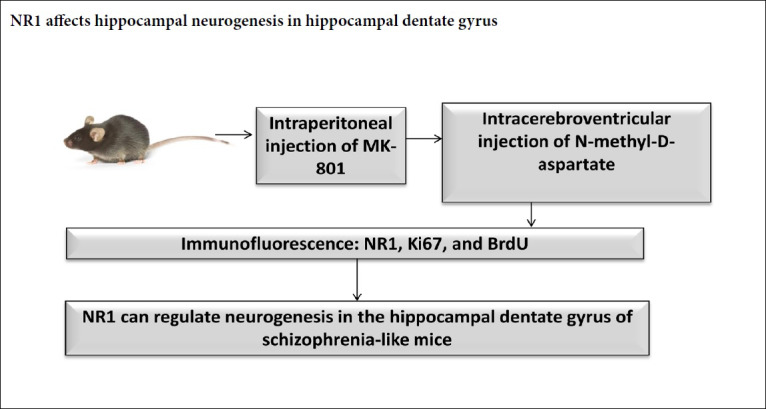
N-methyl-D-aspartate receptor hypofunction is the basis of pathophysiology in schizophrenia. Blocking the N-methyl-D-aspartate receptor impairs learning and memory abilities and induces pathological changes in the brain. Previous studies have paid little attention to the role of the N-methyl-D-aspartate receptor subunit 1 (NR1) in neurogenesis in the hippocampus of schizophrenia. A mouse model of schizophrenia was established by intraperitoneal injection of 0.6 mg/kg MK-801, once a day, for 14 days. In N-methyl-D-aspartate-treated mice, N-methyl-D-aspartate was administered by intracerebroventricular injection in schizophrenia mice on day 15. The number of NR1-, Ki67- or BrdU-immunoreactive cells in the dentate gyrus was measured by immunofluorescence staining. Our data showed the number of NR1-immunoreactive cells increased along with the decreasing numbers of BrdU- and Ki67-immunoreactive cells in the schizophrenia groups compared with the control group. N-methyl-D-aspartate could reverse the above changes. These results indicated that NR1 can regulate neurogenesis in the hippocampal dentate gyrus of schizophrenia mice, supporting NR1 as a promising therapeutic target in the treatment of schizophrenia. This study was approved by the Experimental Animal Ethics Committee of the Ningxia Medical University, China (approval No. 2014-014) on March 6, 2014.
Chinese Library Classification No. R453; R363; R749
Introduction
Schizophrenia is a chronic mental disease (Silva et al., 2017), which leads to poor quality of life and security concerns in society. The underlying biological mechanisms of schizophrenia have yet to be fully understood and are the focus of an active area of neurological research.
Psychiatric diseases such as epilepsy, schizophrenia and autism are caused by an imbalance in brain excitatory and inhibitory chemical transmitters (Levitt, 2005). Glutamate is the main excitatory neurotransmitter. The N-methyl-D-aspartate (NMDA) receptor is the type of glutamate receptor that is involved in synaptic plasticity, learning, memory ability and cognition. NR1 is a crucial functional subunit of the NMDA receptor family (Wu et al., 2018). The NMDA receptor mediates specific features and symptoms associated with schizophrenia which are mediated through alterations in the glutamatergic system (Moghaddam and Javitt, 2012). Non-competitive NMDA receptor antagonists, such as dizocilpine maleate (MK-801), ketamine, and phencyclidine, have been demonstrated to produce behavioral responses that closely resemble neuropsychiatric disorders such as psychosis (Krystal et al., 1994; Lahti et al., 1995; Collo and Merlo, 2018). Administration of these agents to rats has been used to establish animal models for psychosis (Javitt and Zukin, 1991). Experimental models of NMDA receptor hypofunction have been used as the basis of the pathophysiology of schizophrenia (Snyder and Gao, 2019). Blockade of NMDA receptors has also been shown to impair learning and memory abilities as well inducing patho-morphological changes in the brain (Taffe et al., 2002; Riedel et al., 2003). The hippocampus is associated with learning, memory and emotions. Other research and our previous data have indicated that adult neurogenesis occurs in the hippocampus (França, 2018; Schaffner et al., 2018). However, few previous studies have focused on determining the role of NR1 on hippocampal neurogenesis in schizophrenia.
In our previous study, the NMDA-receptor antagonist MK-801 was used to establish a schizophrenia-like mouse model. Expression of NR1 was high in the MK-801 injection group. NMDA injection could reverse the change of NR1 expression (Ding et al., 2017). In this study, we used BrdU and Ki67 to tag the newborn hippocampal neural stem cells and characterize hippocampal neurogenesis. The aim of this study is to investigate the role of NR1 on neurogenesis in the hippocampal dentate gyrus using a schizophrenia-like mouse model.
Materials and Methods
Animals
Forty male specific-pathogen-free C57/BL mice aged 6 weeks old and weighing 18–24 g were provided by Experimental Animal Center of Ningxia Medical University, Yinchuan, China (license No. SCXK (Ning) 2015-0001). The mice were housed in cages at standard room temperature (22 ± 1°C). A circadian light cycle (12-hour light/12-hour dark) was maintained in the housing facilities with food and water freely available. All mice were given 1 week to acclimatize to the housing environment prior to the beginning of experiments. All animal experiments were carried out according to the guidelines for the care and use of the laboratory animals by the Experimental Animal Ethical Committee of the Ningxia Medical University (approval No. 2014-014) on March 6, 2014.
Preparation of MK-801 and NMDA
MK-801 and NMDA were purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in 0.9% saline. MK-801 was used at dose of 0.6 mg/kg and 5 μL NMDA (15 μg/mL) was administered according to our previous work (Ding et al., 2017) and other previous reports (Zuo et al., 2009; Joo et al., 2013; Kruk-Slomka et al., 2016).
Experimental design and drug administration
Male C57/BL mice were randomly divided into four experimental groups (n = 10 per group), consisting of (1) the control group (injected with saline intraperitoneally), (2) the schizophrenia group, (3) the normal saline group (schizophrenia + 0.9% normal saline) and (4) the NMDA group (schizophrenia + NMDA). The mouse schizophrenia model was induced by intraperitoneal injection of MK-801 at a dose of 0.6 mg/kg once daily for 14 days (Kim et al., 2014) in the schizophrenia, normal saline and NMDA groups. Group 3 and 4 mice were anesthetized by intraperitoneal injection of 4% chloral hydrate at 1 mL/100 g body weight. A single hole was drilled into the murine skulls (anteroposterior –0.4 mm from the Bregma, left 1.0 mm from the mediolateral, dorsoventral –2.5 mm) using a stereotaxic apparatus (Narishige, Tokyo, Japan). A single dose of NMDA in saline (5 μL) at a concentration of 15 μg/mL, or an equal volume of 0.9% saline, was injected into the left intracerebroventricular cavity of each mouse only once on day 15 after intraperitoneal injection of MK-801 (0.6 mg/kg) for 14 days in the NMDA or normal saline groups. Mice were intraperitoneally injected with BrdU (Sigma-Aldrich) at a dose of 50 mg/kg 60 hours after being modeled. Twelve hours later, mice were anesthetized with 4% chloral hydrate, and transcardially perfused with phosphate buffered saline and 4% paraformaldehyde. Mice were then decapitated and the brains were fixed with 4% paraformaldehyde and dehydrated through a sucrose gradient of 20% to 30% until the brain sank to the bottom. Brains were frozen and stored at –80°C prior to being cut into 40-μm sections. A schematic outline of the experimental procedure is shown in Figure 1.
Figure 1.
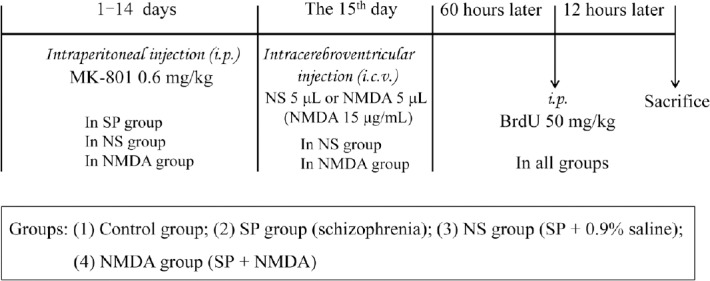
Time process of the experiment.
SP: Schizophrenia; NMDA: N-methyl-D-aspartate; NS: normal saline.
Immunofluorescence
For immunofluorescent staining, brain sections were incubated with a rabbit monoclonal antibody against NR1 (1:500; Abcam, Cambridge, MA, USA), a rat monoclonal antibody against BrdU (1:500; Immunologicals Direct, Oxford Biotechnology Ltd., UK), and a rabbit polyclonal antibody against Ki67 (1:500; Abcam) overnight at 4°C, followed by incubation with an Alexa Fluor 488 labeled goat-anti-rabbit IgG (1:500; Life Technologies Corporation, Gaithersburg, MD, USA) and a Cy3 labeled donkey-anti-rat IgG (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 hour at room temperature. A fluorescence microscope (Fv1000; Olympus, Tokyo, Japan) was used for detection. There were 10 mice in each group, and 10 slices per mouse were chosen from their hippocampal dentate gyrus. The number of immunoreactive cells in each slice was counted and averaged in each group.
Statistical analysis
Measurements are presented as the mean ± SD. Statistical significance was calculated by one-way analysis of variance and least significant difference test. All experiments were conducted at least three times. P values less than 0.05 were considered statistically significant.
Results
NMDA decreases the number of NR1-immunoreactive cells in the hippocampal dentate gyrus of a schizophrenia-like mouse
Compared with the control group, the number of NR1-immunoreactive cells increased in the schizophrenia and normal saline groups (P < 0.05), although no significant difference was detected between the control and NMDA group (P > 0.05). Compared with the schizophrenia and normal saline groups, the number of NR1-immunoreactive cells decreased significantly in the NMDA group (P < 0.05). No significant difference in the number of NR1-immunoreactive cells was found between the schizophrenia and normal saline groups (P > 0.05; Figure 2).
Figure 2.
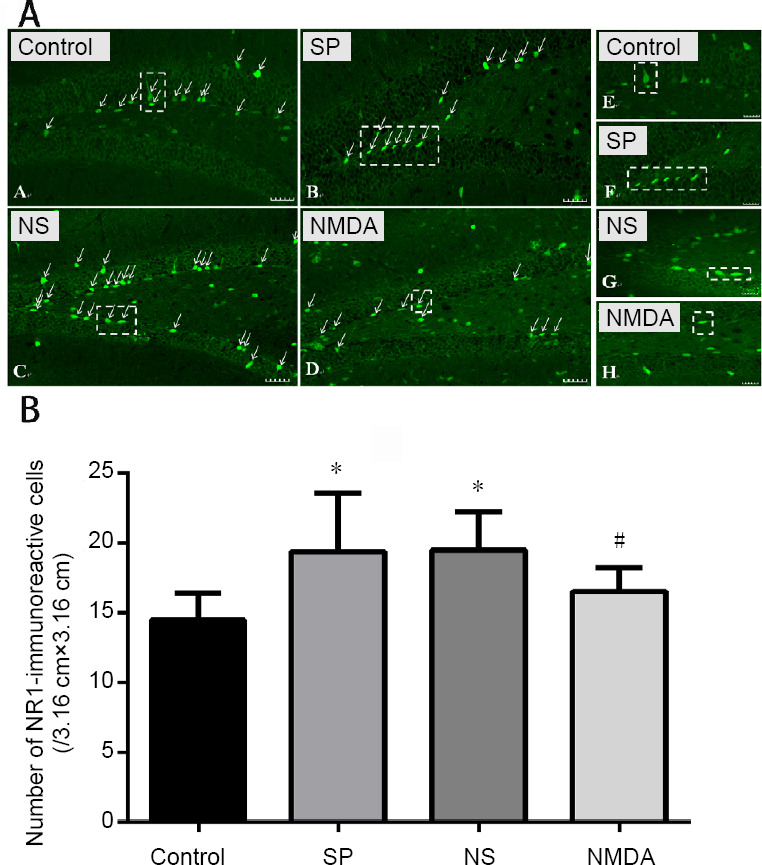
Effect of NMDA on the immunoreactivity of NR1 in the hippocampal dentate gyrus of a schizophrenia-like mouse.
(A) NR1-immunoreactive cell (green, Alexa Fluor 488 labeled) in the hippocampal dentate gyrus. The arrows point to the NR1-immunoreactive cell. Scale bars: 40 μm. The right virtual frame (E–H) is an enlarged version of the left virtual frame (A–D). (B) The number of NR1-immunoreactive cells in the hippocampal dentate gyrus in each group. The histogram data range is the average number of immunoreactive cells in each slice of each group. All data are shown as the mean ± SD (n = 10). *P < 0.05, vs. control group; #P < 0.05, vs. SP or NS group (oneway analysis of variance followed by least significant difference test). CON: Control; SP: schizophrenia; NS: normal saline; NMDA: N-methyl-D-aspartate; NR1: NMDA receptor subunit.
NMDA decreases the number of BrdU-immunoreactive cells in the hippocampal dentate gyrus of a schizophrenia-like mouse
Compared with the control group, the number of BrdU-immunoreactive cells decreased in the schizophrenia and normal saline groups (P < 0.05), and no significant difference was found between the control and NMDA groups (P > 0.05). Compared with the schizophrenia and normal saline groups, the number of BrdU-immunoreactive cells significantly increased in the NMDA group (P < 0.05). However, no significant difference in the number of BrdU-immunoreactive cells was determined between the schizophrenia and normal saline groups (P > 0.05; Figure 3).
Figure 3.
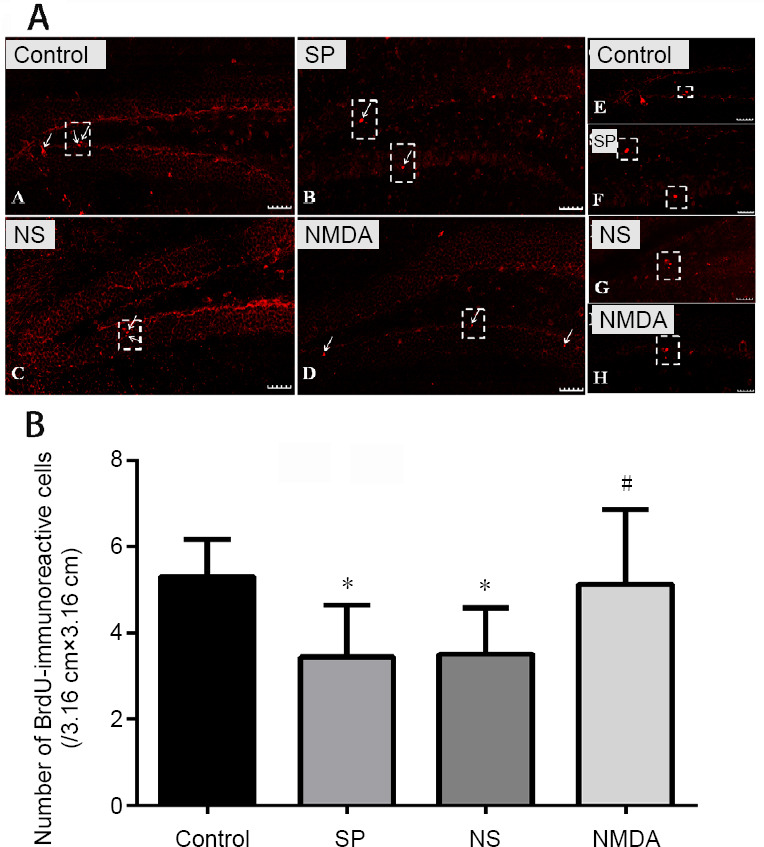
Effect of NMDA on the immunoreactivity of BrdU in the hippocampal dentate gyrus of a schizophrenia-like mouse.
(A) BrdU-immunoreactive cell (red, Cy3 labeled) in the hippocampal dentate gyrus. The arrows point to the BrdU-immunoreactive cell. Scale bars: 40 μm. The right virtual frame (E–H) is an enlarged version of the left virtual frame (A–D). (B) Amount of BrdU-immunoreactive cells in the hippocampal dentate gyrus. The histogram data range is the average number of immunoreactive cells in each slice of each group. All data are shown as the mean ± SD (n = 10; one-way analysis of variance followed by least significant difference test). *P < 0.05, vs. control group; #P < 0.05, vs. SP or NS group. Con: Control; SP: schizophrenia; NS: normal saline; NMDA: N-methyl-D-aspartate.
NMDA decreases the number of Ki67-immunoreactive cells in the hippocampal dentate gyrus of a schizophrenia-like mouse
Compared with the control group, the number of Ki67-immunoreactive cells decreased in the schizophrenia, normal saline and NMDA groups (P < 0.05). Compared with the schizophrenia and normal saline groups, the number of Ki67-immunoreactive cells was significantly greater in the NMDA group (P < 0.05). The number of Ki67-immunoreactive cells increased in the normal saline group compared with the schizophrenia group (P > 0.05; Figure 4).
Figure 4.
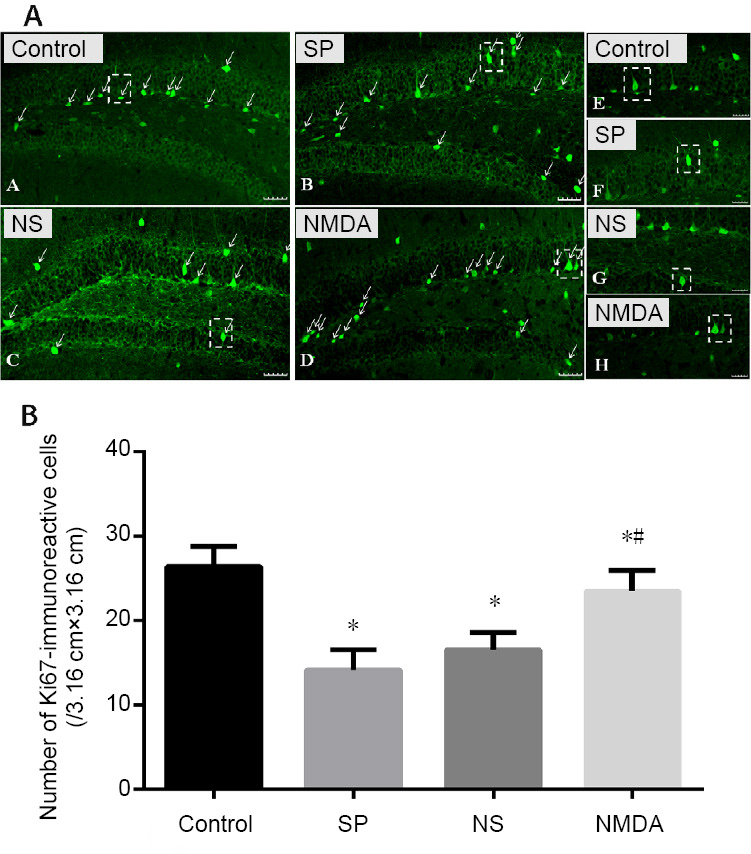
Effect of NMDA on the immunoreactivity of Ki67 in the hippocampal dentate gyrus of a schizophrenia-like mouse.
(A) Ki67-immunoreactive cells (green, Alexa Fluor 488 labeled) in the hippocampal dentate gyrus. The arrows point to the Ki67-immunoreactive cells. Scale bars: 40 μm. The right virtual frame (E–H) is an enlarged version of the left virtual frame (A–D). (B) Amount of Ki67-immunoreactive cells in the hippocampal dentate gyrus. The histogram data range is the average number of immunoreactive cells in each slice of each group. All data are shown as the mean ± SD (n = 10; oneway analysis of variance followed by least significant difference test). *P < 0.05, vs. control group; #P < 0.05, vs. SP or NS group. Con: Control; SP: schizophrenia; NS: normal saline; NMDA: N-methyl-D-aspartate.
Discussion
Current understanding of the etiology and pathogenesis of schizophrenia remains to be fully determined and the present treatment has limited effectiveness. The hippocampus is the key region of the brain associated with learning and memory. Advances in research into the cognitive function of the hippocampus have moved towards its neurogenesis. This process, by which neural stem or progenitor cells generate new neurons, is complex (Emsley et al., 2005). It occurs in most regions of the brain but generally lasts only for a short period after the birth. The lower layer of hippocampal dentate gyrus has an unusual neurogenic ability that can last until adulthood and has been shown to occur throughout a lifetime (Antonelli et al., 2018; Leem et al., 2018). Adult hippocampal neurogenesis is involved in learning and memory (Deng et al., 2010). Some studies found few new neurons, whereas other studies found many new neurons in the adult dentate gyrus (Knoth et al., 2010; Spalding et al., 2013; Dennis et al., 2016; Sorrells et al., 2018). It is now believed that the hippocampus of an adult human continues to generate new neurons (Sorrells et al., 2018). A post-mortem study indicated that abnormal neurogenesis is found in schizophrenics (Ross et al., 2006). This suggests that neurogenesis in the hippocampus may be a potential therapeutic target for the treatment of a number of neural degenerative and other diseases of the central nervous system.
The NMDA receptor is widely expressed in the central nervous system and has been shown to play a crucial role in excitatory synaptic transmission. It is a glutamate-gated ion channel, which is found abundantly in the hippocampus and cerebral cortex. The NMDA receptor is mainly localized in the post-synaptic membrane of nerve cells where it can induce nerve growth, synaptogenesis and maturity of neurons. Excitation of the NMDA receptor acts to promote learning and memory. The NMDA receptor has at least seven subunits, including NR1, NR2A, NR2B, NR2C, NR2D, NR3A and NR3B. Amongst these subunits, NR1 is a key functional subunit of the NMDA receptor family (Stephenson, 2001).
Hypofunction of NMDA receptor activity and the dysregulation of dopaminergic neurotransmission are considered key aspects underlying the mechanism of schizophrenia (Kristiansen et al., 2007). The non-competitive NMDA receptor antagonist MK-801 can induce similar schizophrenic behavior, including both positive and negative symptoms (Meltzer et al., 2013; Brown et al., 2014; Blot et al., 2015). Therefore, the NMDA receptor antagonist MK-801 was used to establish a schizophrenia-like mouse model (Joo et al., 2013; Kruk-Slomka et al., 2016; Ding et al., 2017).
Under many pathological conditions in the nervous system, both chronic and acute NMDA receptor activation can result in neuronal death. Schizophrenia has been shown to make neurons more sensitive to normal levels of NMDA receptor activity, or causes failure in maintaining intra- or intercellular steady state glutamate levels. In acute brain injury such as pain, mechanical injury, stroke and epilepsy disease, glutamate levels are acutely increased and are the leading cause of neuronal death (Liu et al., 2007). In our previous study, MK-801 led to apoptosis of hippocampal neurons and decreased NR1 expression (Ding et al., 2017).
Research by ourselves and others has also shown that the mouse model produces behavioral responses that closely resemble those seen in schizophrenia, along with decreased neurogenesis in the hippocampal dentate gyrus (Yamaguchi et al., 2004; Liu et al., 2006; Tanimura et al., 2009). In this study, very similar results were obtained. BrdU is used to identify dividing cells during mitosis, thus BrdU-immunohistochemistry is used to locate the proliferative cells in the hippocampus of our study (Taupin, 2007). Ki67 expression starts in the G1 phase, remarkably increases in S and G2 phases, reaches a peak in the M phase, disappears in the late split phase, and no expression is found in the G0 phase (Kong et al., 2015). In this study, to investigate alterations of NR1 expression and neurogenesis in the hippocampal dentate gyrus, MK-801 was used to establish a schizophrenia-like mouse model before injection of the NMDA into the lateral ventricle. Our data showed that NR1 expression increased and the number of both BrdU- and Ki67-immunoreactive cells decreased in the schizophrenia and normal saline groups. This up-regulation of NR1 may be due to a compensatory response resulting from glutamatergic deficits by which NMDA can reverse the alterations. NMDA is an agonist of the NMDA receptor. Our data indicated that NMDA inhibits or eliminates the effects of MK-801 on NR1 expression and plays a key role in preventing the neurotoxicity induced by MK-801 in schizophrenia mice. It showed that NR1 is crucial for the regulation of hippocampal neurogenesis and that the NMDA receptor agonist NMDA acted to promote hippocampal neurogenesis in the schizophrenia mice. The effects of MK-801 and NMDA on BrdU and Ki67 observed using immunochemistry also indicated the key role of NR1 in neurogenesis.
Several studies have also reported that glutamatergic neurotransmission can enhance neurogenesis in human neural stem cells (Suzuki et al., 2006; Zhao et al., 2011). One study showed that the NMDA receptor antagonist MK-801 (1.0 mg/kg, intraperitoneally, single administration) stimulated hippocampal adult neurogenesis in adult rats (Gould et al., 1997). The increase in adult neurogenesis by MK-801 (1.0 mg/kg, intraperitoneally, single administration) appeared to be a compensatory effect (Reif et al., 2007). Our study showed hippocampal neurogenesis decreased on administration of MK-801 (0.6 mg/kg, intraperitoneally, daily for 14 days). The paradoxical results might indicate that different doses induce different responses in animals. We have shown that the regulation of hippocampal neurogenesis was associated with NR1 expression. NMDA was shown to have the neuroprotective effect on schizophrenic mice, suggesting that NR1 may be a promising therapeutic target for the treatment of schizophrenia. The limitation of the study was that the NR1 knockout model was not used to study the effect of NR1 on neurogenesis. Further, studies are required to comprehensively understand the underlying signaling pathways mediating NR1 expression and their impact on hippocampal neurogenesis in schizophrenia.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this study.
Financial support: This work was supported by the National Natural Science Foundation of China, No. 81160169 (to JL), 81460214 (to JL), 31660270 (to JD), 31460255 (to JD); the Natural Science Foundation of Ningxia Hui Autonomous Region of China, No. 2018AAC02005 (to JL). The funding sources had no role in study design, conception, analysis or interpretation of data, writing and deciding to submit this paper for publication.
Institutional review board statement: The experimental protocol was approved by the Experimental Animal Ethics Committee of the Ningxia Medical University, China (approval No. 2014-014) on March 6, 2014.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Christopher J. Andrews, University of Queensland, Australia.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81160169 (to JL), 81460214 (to JL), 31660270 (to JD), 31460255 (to JD); the Natural Science Foundation of Ningxia Hui Autonomous Region of China, No. 2018AAC02005 (to JL).
P-Reviewer: Andrews CJ; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Dawes EA, Stow A, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Antonelli F, Casciati A, Tanori M, Tanno B, Linares-Vidal MV, Serra N, Bellés M, Pannicelli A, Saran A, Pazzaglia S. Alterations in morphology and adult neurogenesis in the dentate gyrus of Patched1 heterozygous mice. Front Mol Neurosci. 2018;11:168. doi: 10.3389/fnmol.2018.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blot K, Kimura S, Bai J, Kemp A, Manahan-Vaughan D, Giros B, Tzavara E, Otani S. Modulation of hippocampus-prefrontal cortex synaptic transmission and disruption of executive cognitive functions by MK-801. Cereb Cortex. 2015;25:1348–1361. doi: 10.1093/cercor/bht329. [DOI] [PubMed] [Google Scholar]
- 3.Brown JW, Rueter LE, Zhang M. Predictive validity of a MK-801-induced cognitive impairment model in mice: implications on the potential limitations and challenges of modeling cognitive impairment associated with schizophrenia preclinically. Prog Neuropsychopharmacol Biol Psychiatry. 2014;49:53–62. doi: 10.1016/j.pnpbp.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Collo G, Merlo Pich E. Ketamine enhances structural plasticity in human dopaminergic neurons: possible relevance for treatment-resistant depression. Neural Regen Res. 2018;13:645–646. doi: 10.4103/1673-5374.230288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennis CV, Suh LS, Rodriguez ML, Kril JJ, Sutherland GT. Human adult neurogenesis across the ages: An immunohistochemical study. Neuropathol Appl Neurobiol. 2016;42:621–638. doi: 10.1111/nan.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding J, Zhou HH, Ma QR, He ZY, Ma JB, Liu YM, Zhang YW, He YQ, Liu J. Expression of NR1 and apoptosis levels in the hippocampal cells of mice treated with MK801. Mol Med Rep. 2017;16:8359–8364. doi: 10.3892/mmr.2017.7674. [DOI] [PubMed] [Google Scholar]
- 8.Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 9.França TFA. The evolutionary significance of hippocampal neurogenesis. Eur J Neurosci. 2018;48:2945–2947. doi: 10.1111/ejn.14144. [DOI] [PubMed] [Google Scholar]
- 10.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 12.Joo J, Lee S, Nah SS, Kim YO, Kim DS, Shim SH, Hwangbo Y, Kim HK, Kwon JT, Kim JW, Song HY, Kim HJ. Lasp1 is down-regulated in NMDA receptor antagonist-treated mice and implicated in human schizophrenia susceptibility. J Psychiatr Res. 2013;47:105–112. doi: 10.1016/j.jpsychires.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Kim TW, Kang HS, Park JK, Lee SJ, Baek SB, Kim CJ. Voluntary wheel running ameliorates symptoms of MK-801-induced schizophrenia in mice. Mol Med Rep. 2014;10:2924–2930. doi: 10.3892/mmr.2014.2644. [DOI] [PubMed] [Google Scholar]
- 14.Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5:e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong L, Hu Y, Yao Y, Jiao Y, Li S, Yang J. The coumarin derivative osthole stimulates adult neural stem cells, promotes neurogenesis in the hippocampus, and ameliorates cognitive impairment in APP/PS1 transgenic mice. Biol Pharm Bull. 2015;38:1290–1301. doi: 10.1248/bpb.b15-00142. [DOI] [PubMed] [Google Scholar]
- 16.Kristiansen LV, Huerta I, Beneyto M, Meador-Woodruff JH. NMDA receptors and schizophrenia. Curr Opin Pharmacol. 2007;7:48–55. doi: 10.1016/j.coph.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Kruk-Slomka M, Budzynska B, Slomka T, Banaszkiewicz I, Biala G. The influence of the CB1 receptor ligands on the schizophrenia-like effects in mice induced by MK-801. Neurotox Res. 2016;30:658–676. doi: 10.1007/s12640-016-9662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 19.Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 20.Leem YH, Kato M, Chang H. Regular exercise and creatine supplementation prevent chronic mild stress-induced decrease in hippocampal neurogenesis via Wnt/GSK3beta/beta-catenin pathway. J Exerc Nutrition Biochem. 2018;22:1–6. doi: 10.20463/jenb.2018.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levitt P. Disruption of interneuron development. Epilepsia. 2005;7(46 Suppl):22–28. doi: 10.1111/j.1528-1167.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Suzuki T, Seki T, Namba T, Tanimura A, Arai H. Effects of repeated phencyclidine administration on adult hippocampal neurogenesis in the rat. Synapse. 2006;60:56–68. doi: 10.1002/syn.20275. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meltzer HY, Rajagopal L, Huang M, Oyamada Y, Kwon S, Horiguchi M. Translating the N-methyl-D-aspartate receptor antagonist model of schizophrenia to treatments for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol. 2013;16:2181–2194. doi: 10.1017/S1461145713000928. [DOI] [PubMed] [Google Scholar]
- 25.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reif A, Schmitt A, Fritzen S, Lesch KP. Neurogenesis and schizophrenia: dividing neurons in a divided mind? Eur Arch Psychiatry Clin Neurosci. 2007;257:290–299. doi: 10.1007/s00406-007-0733-3. [DOI] [PubMed] [Google Scholar]
- 27.Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- 28.Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Schaffner I, Minakaki G, Khan MA, Balta EA, Schlotzer-Schrehardt U, Schwarz TJ, Beckervordersandforth R, Winner B, Webb AE, DePinho RA, Paik J, Wurst W, Klucken J, Lie DC. FoxO function is essential for maintenance of autophagic flux and neuronal morphogenesis in adult neurogenesis. Neuron. 2018;99:1188–1203.e6. doi: 10.1016/j.neuron.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva RDC, Albuquerque SGC, Muniz AV, Filho PPR, Ribeiro S, Pinheiro PR, Albuquerque VHC. Reducing the schizophrenia stigma: a new approach based on augmented reality. Comput Intell Neurosci. 2017;2017:2721846. doi: 10.1155/2017/2721846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder MA, Gao WJ. NMDA receptor hypofunction for schizophrenia revisited: Perspectives from epigenetic mechanisms. Schizophr Res. 2019 doi: 10.1016/j.schres.2019.03.010. doi:101016/jschres201903010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, Gutierrez AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z, Alvarez-Buylla A. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephenson FA. Subunit characterization of NMDA receptors. Curr Drug Targets. 2001;2:233–239. doi: 10.2174/1389450013348461. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki M, Nelson AD, Eickstaedt JB, Wallace K, Wright LS, Svendsen CN. Glutamate enhances proliferation and neurogenesis in human neural progenitor cell cultures derived from the fetal cortex. Eur J Neurosci. 2006;24:645–653. doi: 10.1111/j.1460-9568.2006.04957.x. [DOI] [PubMed] [Google Scholar]
- 36.Taffe MA, Davis SA, Gutierrez T, Gold LH. Ketamine impairs multiple cognitive domains in rhesus monkeys. Drug Alcohol Depend. 2002;68:175–187. doi: 10.1016/s0376-8716(02)00194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanimura A, Liu J, Namba T, Seki T, Matsubara Y, Itoh M, Suzuki T, Arai H. Prenatal phencyclidine exposure alters hippocampal cell proliferation in offspring rats. Synapse. 2009;63:729–736. doi: 10.1002/syn.20660. [DOI] [PubMed] [Google Scholar]
- 38.Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Wu C, Wang J, Guo X, Zhang Y. Ketamine exacerbates cortical neuroapoptosis under hyperoxic conditions by upregulating expression of the N-methyl-D-aspartate receptor subunit NR1 in the developing rat brain. BMC Anesthesiol. 2018;18:52. doi: 10.1186/s12871-018-0511-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamaguchi M, Suzuki T, Seki T, Namba T, Juan R, Arai H, Hori T, Asada T. Repetitive cocaine administration decreases neurogenesis in adult rat hippocampus. Ann N Y Acad Sci. 2004;1025:351–362. doi: 10.1196/annals.1316.043. [DOI] [PubMed] [Google Scholar]
- 41.Zhao L, Jiao Q, Yang P, Chen X, Zhang J, Zhao B, Zheng P, Liu Y. Metabotropic glutamate receptor 5 promotes proliferation of human neural stem/progenitor cells with activation of mitogen-activated protein kinases signaling pathway in vitro. Neuroscience. 2011;192:185–194. doi: 10.1016/j.neuroscience.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 42.Zuo DY, Cao Y, Zhang L, Wang HF, Wu YL. Effects of acute and chronic administration of MK-801 on c-Fos protein expression in mice brain regions implicated in schizophrenia with or without clozapine. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:290–295. doi: 10.1016/j.pnpbp.2008.12.002. [DOI] [PubMed] [Google Scholar]


