Figure 1.
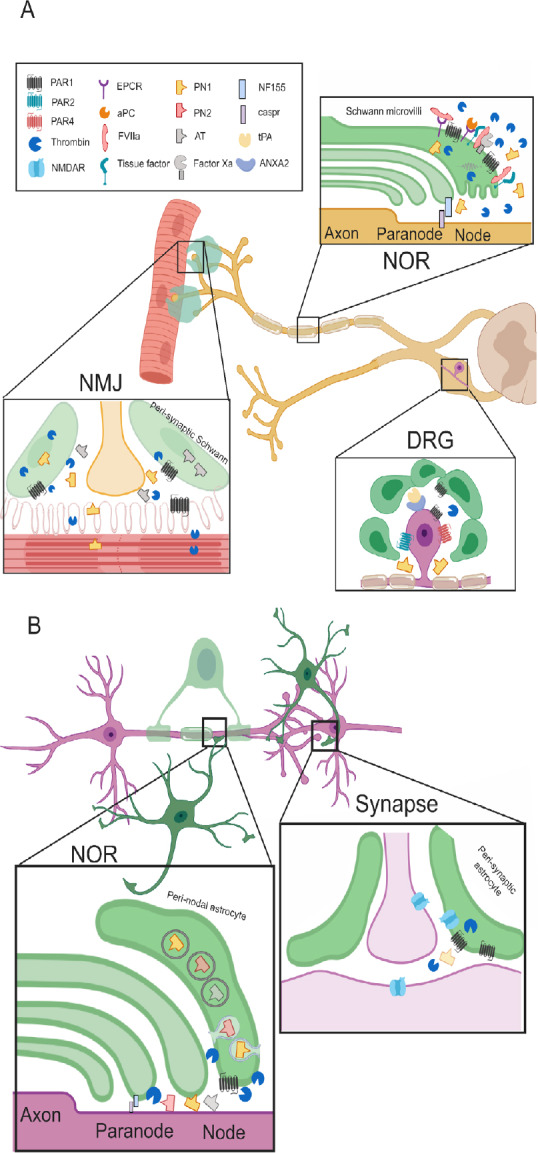
The neuro-glial coagulonome (NG-coagulonome) in the peripheral nervous system (PNS) and central nervous system (CNS).
(A) The NG-coagulonome in the PNS: Schwann cells modulate nerve function in the PNS by secretion of coagulation factors and inhibitors and expression of corresponding receptors. NOR: Schwann cells microvilli at the NOR express protease activating receptor 1 (PAR1). High levels of thrombin activate PAR1 and cause conduction block, whereas low levels activate PAR1 via the endothelial protein C receptor (EPCR) pathway, inducing a neuroprotective phenotype in Schwann cells. Tissue factor binds FVIIa which cleave FX to generate FXa. Levels of FXa and activated protein C (aPC) rises after injury, modulate activation of prothrombin to thrombin. The Schwann secretes PN1 in electrical activity dependent manner which inhibits thrombin. Thrombin inhibition supports neuroregenerative processes. FVIIa potentially binds EPCR as well, suggesting fine balance between the different modes of PAR1 activation. Myelin expresses the adhesion protein neurofascin 155 (NF155) which connects it to the axons via caspr. This structure is disturbed by thrombin. DRG: Satellite cells at the DRG express PAR1 and neurons express PAR1, 2 and 4. PAR1 is elevated in diseases involving peripheral neuropathy such as human immunodeficiency virus (HIV). The complex of tissue plasminogen activator (tPA) and annexin A2 (ANXA2) is expressed on the DRG cells and associated with chronic pain. This suggests a local production of plasmin which is another PAR1 activating protease. Thrombin is inhibited by PN1 which is secreted from glia cells and counteracts the detrimental PAR1 activation. NMJ: Both muscle fibers and Schwann express PAR1 and secrete thrombin. Electrical activity induces thrombin secretion which leads to synaptic elimination. This is prevented by secretion of the thrombin inhibitors such as anti-thrombin (AT) and PN1 from glia cells mainly. (B) The known NG-coagulonome in the CNS: Astrocytes modulate nerve conduction in the CNS at the NOR and at the synapse. NOR: Peri-nodal astrocytes express PAR1, implying its role in nerve conduction. Thrombin cleaves NF155 thus, induces neurodegeneration. Protease nexin (PN)1 is secreted from astrocytes by exocytosis and modulate thrombin depended proteolysis of NF155. PN2 and AT are assumed to be secreted from astrocytes as well. These thrombin inhibitors are part of a feedback loop opposing thrombin damage seen in disease models such as experimental autoimmune encephalomyelitis (EAE). Synapse: Astrocyte’s PAR1 activation by thrombin depolarizes the synapse, whereas depolarization itself releases thrombin from the astrocyte. High levels of thrombin following injury activates PAR1 and lead to the secretion of glutamate, thus activating N-methyl-D-aspartic acid receptor (NMDAR). NMDAR is localized to all of the three partite synapse components. Its activation creates slow long term potentiation (LTP) which changes synaptic plasticity. NOR: node of Ranvier; DRG: dorsal root ganglia; NMJ: neuromuscular junction. Created with BioRender.com.
