Life expectancy in industrialized and developed countries will continue to increase in the near future. Consequently, over the last years, the incidence and social impact of neurodegenerative diseases have increased, highlighting an urgent need for new and more effective therapeutic strategies to counter these terrible disorders. While we tend to think about neurodegenerative diseases as conditions that are uniquely associated with the elder age, these diseases cover a diverse range across the entire lifespan, even affecting infants and children.
The umbrella term neurodegenerative diseases stands for an ensemble of conditions primarily affecting the neurons in the human brain, which frequently culminate in the death of the aforementioned cells and consequent loss of related functionalities. Examples of neurodegenerative diseases are Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease, amyotrophic lateral sclerosis (ALS), frontotemporal lobar dementia (FTD) and a diverse range of other pathological conditions. Even if these diseases are different in their pathophysiology, they are all accompanied by taking away a person’s independence, cognitive capacities, personality, and sense of self. At the molecular level, the causes of neurodegeneration are still unclear, complicating the identification of early biomarkers that may anticipate the disease onset, thus improving treatments outcomes. This limited knowledge of pathological processes makes, therefore, the identification of molecular targets for the rational development of drugs very difficult, but at the same time challenging. For example, an aspect characterizing the onset of several neurodegenerative diseases, such as AD, PD or ALS, is the accumulation of misfolded, ubiquitinated and hyperphosphorylated proteins in the central nervous system, a phenomenon called proteinopathy. Dependently by the nature of the proteins involved, it is possible to differentiate several classes of disorders, such as amyloidopathies, tauopathies or synucleopathies. The spectrum of neurodegenerative disorders involving the transactive response DNA binding protein of 43 kDa (TDP-43) takes the name of TDP-43 proteinopathies. ALS and FTD are the main pathological conditions associated with TDP-43 proteinopathies: it worth noting that ALS is a multifactorial disease, which can be distinguished in a sporadic form, affecting the majority of the population and characterized by proteinopathies related manifestations, and in an familiar form, concerning a minor group of patients in which different genes alterations have been mapped (SOD1, C9orf72, FUS).
In detail, TDP-43 is a DNA/RNA binding protein that, in physiological conditions, is predominantly localized in the cell nucleus, where it contributes to controlling the transcription, transport, maturation, and stability of different types of RNA. Under pathological conditions, TDP-43 is hyperphosphorylated and cleaved to generate C-terminal fragments of 24–26 kDa, which accumulate in the affected brain and spinal cord regions. The molecular basis for TDP-43 proteolysis and C-terminal fragments generation remains largely unknown, although it is reasonable to believe that many TDP-43 truncation products are linked to TDP-43 proteinopathies. Nowadays it is clear that the intracellular aggregates of phosphorylated TDP-43 are a major component of the ubiquitin-positive inclusions detected in ALS/FTD patients’ brains and thus can be identified as specific pathological hallmarks. In addition to the primary role played by TDP-43 in the onset of ALS, other pathological mechanisms take part, most of which are still unclear. For example, the high level of excitatory amino acid glutamate found in patients cerebrospinal fluid has led to the hypothesis of motoneurons damage mediated by excitotoxicity. The pathological importance of glutamate homeostasis was supported by the approval in 1995 of the drug riluzole, a benzothiazole compound, which chemical structure is reported in Figure 1B, characterized by a mechanism of action that has remained until now quite elusive. Initially, riluzole was classified as a modulator of glutamatergic communication, even if a direct interaction with the glutamate receptors has never been established (Doble, 1996). Regarding the aberrant phosphorylation of TDP-43, protein kinase CK1 isoform δ (CK1δ) has been identified as the main player able to phosphorylate the keys residues Ser379, Ser403/4, and Ser409/410, promoting TDP-43 mislocalization and, consequently, neurons death (Nonaka et al., 2016). As already anticipated, ALS and FTD are the main pathological conditions associated with TDP-43 proteinopathies and, although the clinical manifestations are different, about 20% ALS patients also develop FTD and vice versa (Katz and Woolley, 2019).
Figure 1.
Unmasking the molecular link between riluzole and protein kinase CK1δ.
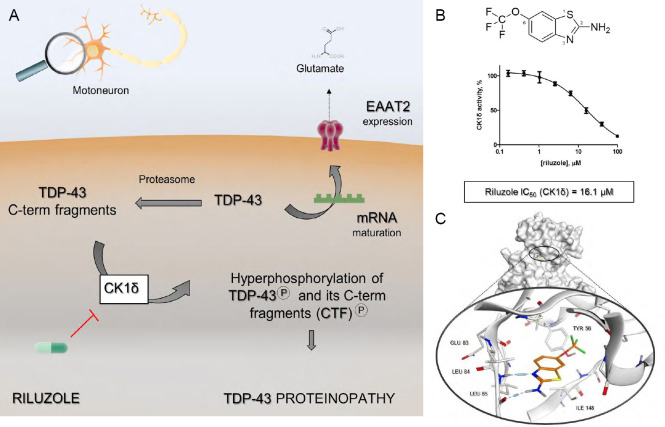
(A) Riluzole revisited mechanism of action: the aberrant phosphorylation of the full-length nuclear protein TDP-43 and its C-term fragments (CTF) generated by proteasome complex, is modulated through the inhibition of CK1δ catalytic activity. As a result, riluzole prevents the cascade of events that culminates with TDP-43 compartmentalization at the cytoplasmic level. The maintenance of TDP-43 homeostasis assured by riluzole allows the correct maturation of specific mRNA transcripts and therefore the normal expression of the EATA2 transporter, which prevents the lethal phenomenon of excitotoxicity. (B) the chemical structure of 1,6-(trifluoromethoxy)-2-benzothiazolamine, commonly known as riluzole. The IC50 value for protein kinase CK1δ inhibition, quantify through a biochemical assay, has been reported, to underline the link between CK1δ and ALS treatment. (C) The proposed binding mode of drug riluzole inside CK1δ catalytic site, predicted through molecular docking experiments. ALS: Amyotrophic lateral sclerosis; CK1δ: protein kinase CK1 isoform δ; TDP-43: transactive response DNA binding protein of 43 kDa; EAAT2: excitatory amino acid transporter-2.
Very recently, using a combination of in silico and experimental approaches, it has been described for the first time the micromolar inhibitory activity of riluzole toward the protein kinase CK1δ (Bissaro et al., 2018). A putative binding mode of riluzole within the kinase ATP binding site, predicted through molecular docking experiments, is shown in Figure 1C. This finding allowed us not only to deorphanize riluzole but also to rethink its mechanism of action, linking together the two main clinical symptoms of ALS: glutamate-mediated excitotoxicity and TDP-43 related proteinopathy. As summarized by Figure 1A, riluzole, that is a brain permeant molecule, through the inhibition of CK1δ catalytic activity could prevent TDP-43 hyperphosphorylation, which represents a key step in the mechanism of aberrant protein accumulation into the cytoplasm. Since, as it has been previously demonstrated, TDP-43 plays a crucial role in specific mRNAs maturations, the loss of its physiological localization can be linked to the impaired expression of the excitatory amino acid transporter‐2 on glial cells and, thus, to a pathological accumulation of glutamate in nervous tissues. (Rosenblum and Trotti, 2017).
After more than twenty years during which riluzole was used as the only therapeutic approach for ALS treatment without understanding its exact mechanism of action, this discovery has therefore made possible to highlight the critical role played by protein CK1δ, both from a pathological and pharmacological point of view, paving the way for the development of more powerful treatment. Many are, as a consequence, the questions and perspectives that open about the role of this protein kinase in TDP-43 related proteinopathies and ALS treatment, of which the most interesting will be introduced below.
Riluzole: a drug with a mysterious mechanism of action: In the 1950s, a number of 2-benzothiazolamines were intensively investigated as central muscle relaxants as reported, for example, by Domino et al. (1952). At the beginning of these studies, the pharmaceutical interest in this class of chemical compounds was limited, as long as the pharmacological profile of riluzole was not clearly described. Riluzole (1,6-(trifluoromethoxy)-2-benzothiazolamine, PK 26124, RP 54274, Rilutek) was found to interfere with glutamate neurotransmission in biochemical, electrophysiological, and behavioral experiments. Interestingly, in the first biochemical models, riluzole antagonized the release of acetylcholine-induced by N-methyl-D-aspartate in vitro and blocked the increase in cyclic guanosine monophosphate levels in the cerebellar cortex induced by glutamate in vitro and in vivo (Benavides et al., 1985). In addition, electrophysiological studies have shown riluzole to interfere with glutamatergic transmission at both the pre- and postsynaptic level, even if it did not act as a competitive antagonist at N-methyl-D-aspartate type receptors (Koek and Woods, 1988). Finally, it has also been demonstrated that riluzole is a highly specific blocker of inactivated Na+ channels, which in turn inhibit glutamate release. Riluzole has been shown to have interesting neuroprotective effects in vitro, protecting primary cultures of rat cortical neurons against anoxic stress and against an excitotoxic factor present in cerebrospinal fluid from ALS patients. Furthermore, riluzole ability in protecting wobbler mouse, one of the most useful models of motoneurons degeneration, from glutamate excitotoxic effects was compared with that provided by the AMPA receptor antagonist RPR119990. Riluzole has been shown to increase motor behavior in the rodent, due to a diminished motoneurons loss (Fumagalli et al., 2006). Moreover, clinical studies have also demonstrated that riluzole slows disease progression in ALS patients (Bensimon et al., 1994). Following pivotal clinical trials in ALS, approval of riluzole by the US Food and Drug Administration in 1995 was met with great optimism. It has been marketed in dozens of countries around the world since the late 1990s, and generic versions became available in the U.S. in 2013. Despite being associated with a short survival benefit of 2–3 months equating to a 9% increase in 1-year survival, the subsequent adoption of riluzole as a treatment for ALS was perhaps reflective of a desperate need for therapeutic options in the face of this devastating progressive disease (Dharmadasa and Kiernan, 2018). More than two decades after riluzole was first approved for ALS, a more efficacious treatment is yet to be discovered and, together with the recently approved drug edaravone, riluzole still represents the only pharmacological treatment available against ALS. As previously described, despite increasing scientific rhetoric on the subject, the mechanism of therapeutic benefit afforded by riluzole remains undetermined. Several pathways have been postulated, ranging from central anti-glutaminergic modulation of excitotoxic pathways, mitochondrial function, and changes to fat metabolism, to peripheral axonal effects on persistent sodium channel function and potentiation of calcium-dependent potassium currents. Moreover, the uncertainty about its molecular mechanism of action has made slower and complicated the development of more powerful and effective drug candidates. For all the aforementioned reasons, the recent discovery linking the action of riluzole with the inhibition of the protein kinase CK1δ allows not only to better understand its mechanism but also to highlight the importance of this protein target in the treatment of ALS and, more generally, TDP-43 related proteinopathies. The limited micromolar inhibitory potency of riluzole, as reported in Figure 1B, makes it more similar to a hit candidate than a mature drug and could be one of the reasons behind its limited therapeutics efficacy and, therefore, anticipates an interesting question. Will brain permeant small molecules able to inhibit the catalytic activity of protein kinase CK1δ in a more potent and, eventually, a more selective way be able to improve the poor pharmacological profile of riluzole in ALS treatment? A first encouraging confirmation is provided by a study conducted by Martinez and Perez, in which low nanomolar CK1δ inhibitors showed to efficiently modulate TDP-43 hyperphosphorylation (Salado et al., 2014). From a perspective point of view, it becomes therefore fundamental to evaluate if CK1δ inhibitors are also able to prevent TDP-43 aberrant cytoplasmic segregation and if this, as hypothesized, could result in a restoration of the physiological glutamatergic signaling, due to normal expression of the excitatory amino acid transporter‐2 transporter.
Riluzole and protein kinase CK1δ: not only TDP-43 proteinopathies: If the information concerning riluzole is seen within the scientific literature, curiously emerges how this molecule has long been investigated not only in the context of ALS but also to treat other diseases characterized by proteinopathies, as AD or PD. Although the results of such studies are often contradictory and not easy to interpret, it is difficult in light of the latest discoveries not to speculate on the possibility that the protein CK1δ could represent a link in the pharmacological treatment of different neurodegenerative pathologies. CK1δ is, in fact, able to recognize as a substrate and therefore phosphorylate both the tau and α-synuclein proteins, which represent a key process in the onset of tauopathies (AD) and synucleopathies (PD), respectively. This finding supports the hypothesis that riluzole, and more generally all the inhibitors of the protein kinase CK1δ, could represent interesting pharmacological probes in a wider spectrum of neurodegenerative diseases, all united by an aberrant phosphorylation process responsible for proteinopathies onset.
We are very grateful to Chemical Computing Group, OpenEye, and Acellera for the scientific and technical partnership, and we thanks for NVIDIA Corporation with the donation of the Titan V GPU used to perform the computational studies.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Benavides J, Camelin JC, Mitrani N, Flamand F, Uzan A, Legrand JJ, Gueremy C, Le Fur G. 2-Amino-6-trifluoromethoxy benzothiazole, a possible antagonist of excitatory amino acid neurotransmission-- II. Biochemical properties. Neuropharmacology. 1985;24:1085–1092. doi: 10.1016/0028-3908(85)90196-0. [DOI] [PubMed] [Google Scholar]
- 2.Bensimon G, Lacomblez L, Meininger V Group the AS. A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 3.Bissaro M, Federico S, Salmaso V, Sturlese M, Spalluto G, Moro S. Targeting protein kinase CK1δ with riluzole: could it be one of the possible missing bricks to interpret its effect in the treatment of ALS from a molecular point of view? ChemMedChem. 2018;13:2601–2605. doi: 10.1002/cmdc.201800632. [DOI] [PubMed] [Google Scholar]
- 4.Dharmadasa T, Kiernan MC. Riluzole, disease stage and survival in ALS. Lancet Neurol. 2018;17:385–386. doi: 10.1016/S1474-4422(18)30091-7. [DOI] [PubMed] [Google Scholar]
- 5.Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233–241. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- 6.Domino EF, Unna KR, Kerwin J. Pharmacological properties of benzazoles. I. Relationship between structure and paralyzing action. J Pharmacol Exp Ther. 105:486–497. [PubMed] [Google Scholar]
- 7.Fumagalli E, Bigini P, Barbera S, De Paola M, Mennini T. Riluzole, unlike the AMPA antagonist RPR119990, reduces motor impairment and partially prevents motoneuron death in the wobbler mouse, a model of neurodegenerative disease. Exp Neurol. 2006;198:114–128. doi: 10.1016/j.expneurol.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Katz JS, Woolley SC. Physician's Field Guide to Neuropsychology. Springer New York: New York, NY: 2019. Amyotrophic Lateral Sclerosis. [Google Scholar]
- 9.Koek W, Woods JH. 2-Amino-6-trifluoromethoxy benzothiazole (PK 26124), a proposed antagonist of excitatory amino acid neurotransmission, does not produce phencyclidine-like behavioral effects in pigeons, rats and rhesus monkeys. Neuropharmacology. 1988;27:771–775. doi: 10.1016/0028-3908(88)90089-5. [DOI] [PubMed] [Google Scholar]
- 10.Nonaka T, Suzuki G, Tanaka Y, Kametani F, Hirai S, Okado H, Miyashita T, Saitoe M, Akiyama H, Masai H. Phosphorylation of TAR DNA-binding protein of 43 kDa (TDP-43) by truncated casein kinase 1δ triggers mislocalization and accumulation of TDP-43. J Biol Chem. 2016;291:5473–5483. doi: 10.1074/jbc.M115.695379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenblum LT, Trotti D. In advances in neurobiology. Vol. 16. Springer, Cham: 2017. EAAT2 and the molecular signature of amyotrophic lateral sclerosis; pp. 117–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salado IG, Redondo M, Bello ML, Perez C, Liachko NF, Kraemer BC, Miguel L, Lecourtois M, Gil C, Martinez A. Protein kinase CK-1 inhibitors as new potential drugs for amyotrophic lateral sclerosis. J Med Chem. 2014;57:2755–2772. doi: 10.1021/jm500065f. [DOI] [PMC free article] [PubMed] [Google Scholar]