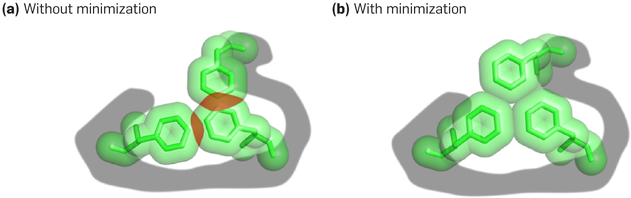
Figure 2. Favorable conformation.
(a) A conformation modeled using ideal rotamers may have steric clashes—atom pairs that are unphysically close together—even when (b) continuous minimization of the conformation’s energy, without changing the rotamers of any residues, results in a very favorable energy. This underscores the need to account for continuous flexibility throughout sequence and conformational search for protein design.
Figure adapted with permission from Gainza et al.9