Abstract
Campylobacter jejuni, a common foodborne zoonotic pathogen, causes gastroenteritis worldwide and is increasingly resistant to antibiotics. We aimed to investigate the antimicrobial resistance (AMR) genotypes of C. jejuni isolated from humans, poultry and birds from wild and urban Italian habitats to identify correlations between phenotypic and genotypic AMR in the isolates. Altogether, 644 C. jejuni isolates from humans (51), poultry (526) and wild- and urban-habitat birds (67) were analysed. The resistance phenotypes of the isolates were determined using the microdilution method with EUCAST breakpoints, and AMR-associated genes and single nucleotide polymorphisms were obtained from a publicly available database. Antimicrobial susceptibility testing showed that C. jejuni isolates from poultry and humans were highly resistant to ciprofloxacin (85.55% and 76.47%, respectively), nalidixic acid (75.48% and 74.51%, respectively) and tetracycline (67.87% and 49.02%, respectively). Fewer isolates from the wild- and urban-habitat birds were resistant to tetracycline (19.40%), fluoroquinolones (13.43%), and quinolone and streptomycin (10.45%). We retrieved seven AMR genes (tet (O), cmeA, cmeB, cmeC, cmeR, blaOXA-61 and blaOXA-184) and gyrA-associated point mutations. Two major B-lactam genes called blaOXA-61 and blaOXA-184 were prevalent at 62.93% and 82.08% in the poultry and the other bird groups, respectively. Strong correlations between genotypic and phenotypic resistance were found for fluoroquinolones and tetracycline. Compared with the farmed chickens, the incidence of AMR in the C. jejuni isolates from the other bird groups was low, confirming that the food-production birds are much more exposed to antimicrobials. The improper and overuse of antibiotics in the human population and in animal husbandry has resulted in an increase in antibiotic-resistant infections, particularly fluoroquinolone resistant ones. Better understanding of the AMR mechanisms in C. jejuni is necessary to develop new strategies for improving AMR programs and provide the most appropriate therapies to human and veterinary populations.
Introduction
Campylobacter jejuni infections are one of the most prevalent and widespread causes of bacterial diarrhoeal disease in humans. Over the last 10 years, the incidence and prevalence of campylobacteriosis has increased in both developed and developing countries, with about 500 million cases of gastroenteritis reported annually [1]. In the European Union, campylobacteriosis is considered the most frequent foodborne infection, with more than 240,000 confirmed human cases per year [2]. Most of the cases are self-limiting with symptoms such as fever, abdominal cramping and bloody diarrhoea. Rarely, the infection might lead to post-infectious neurological complications including Guillain-Barrè and Miller-Fischer syndromes. Campylobacter infections can also predispose people to gastrointestinal autoimmune disorders like celiac disease and inflammatory bowel disease [3]. Campylobacter transmission occurs mainly from exposure to farm animals with such infections, with subsequent passage through the food chain to retail food products [4, 5]. Poultry animals are considered the major infection reservoir and humans most frequently become infected by handling raw, contaminated chicken and turkey meat [4, 6]. Other food products, including beef, pork, lamb, unpasteurized milk, untreated water and seafood are also considered risk factors for campylobacteriosis [7–9]. Although Campylobacter enteritis is self-limiting and antibiotic treatment is usually not indicated [10], in some cases the illness can progress to bacteraemia or become an extraintestinal infection and require antimicrobial therapy, especially in immunocompromised patients [11, 12]. In such cases, the drugs of choice are macrolides and fluoroquinolones, the latter of which is the last class of antimicrobials in common use for treating all diarrheal illnesses, including traveller’s diarrhoea. However, over-use of antimicrobials in the human population and in food animals has increased the number of antibiotic-resistant infections, especially fluoroquinolone-resistant ones [13]. This is a problem because campylobacteriosis is clinically indistinguishable from the gastrointestinal infections caused by other bacterial pathogens. Consequently, the empirical use of fluoroquinolones for treating gastrointestinal infections promotes antibiotic resistance to this class of molecules.
Tetracycline and beta-lactam antimicrobials are also used to treat intestinal infections but they are not generally recommended for treating Campylobacter infections [14–17]. Gentamycin, however, shows potent in vitro activity and may be considered as an alternative treatment. C. jejuni is naturally transformable, making the acquisition of antibiotic resistant genes from other organisms likely [13]. The genetic determinants of antibiotic resistance in C. jejuni, which are chromosomally or plasmid encoded, comprise both endogenous and acquired genes [13]. In general, the different antibiotic resistance mechanisms can be summarised as follows: modification of the antimicrobial target and/or its expression (e.g., DNA gyrase mutations), inability of the antibiotic to reach its target (e.g., upregulated expression of the major outer membrane protein), antibiotic efflux (e.g., multidrug efflux pumps such as CmeABC) and modification or inactivation of the antibiotic (e.g., beta-lactam production) [13]. The different mechanisms involved in antibiotic resistance in C. jejuni are often synergic. Trends in antimicrobial resistance (AMR) have shown a clear correlation related to the use of antibiotics in the animal production industry, and antibiotic-resistant Campylobacter strains have been isolated from humans [17]. Some studies have supported the hypothesis that resistance patterns in poultry could be used as predictors of human resistance patterns, particularly for fluoroquinolones [18, 19]. The increment of resistant strains to commonly used antibiotics in campylobacteriosis makes it necessary the research a more reliable methods in order to investigate antimicrobial susceptibility as well as alternatives therapies [16].
These indications emphasizes the need for improved surveillance and data sharing, confirming the importance of rapid and reproducible methods predicting resistance phenotypes and defining resistance mechanism for surveillance diagnostics. The objectives of this study were to identify AMR genotypes in C. jejuni isolated from humans, poultry and birds from wild and urban environments, and to assess whether any correlations exist between phenotypic and genotypic resistance in the isolates.
Materials and methods
Sample selection and experimental design
Altogether, 644 C. jejuni strains from the collection at the National Reference Laboratory for Campylobacter (NRL, http://www.izs.it/IZS/Eccellenza/Centri_nazionali/LNR_-_Campylobacter) were selected for this study. The collection comprises 51 strains isolated from humans, 246 isolated from retail chicken meat, 280 strains isolated from broiler chickens and 67 wildlife strains isolated from birds living in wild and urban habitats. All the strains were isolated by different monitoring and surveillance plans. The human isolates were from acute campylobacteriosis cases collected in Italy during 2015–2017. The food-related isolates were obtained from a nationwide monitoring study during the one-year period from 2015–2016. The isolates from farmed animals, which came from another nationwide monitoring plan, represent 85% of the intensive broiler production facilities in Italy during the one year period from 2015–2016. The wildlife strains were isolated via passive surveillance monitoring by the Istituti Zooprofilattici Sperimenatali (IIZZSS) network during 2015–2017. The C. jejuni isolates from two greenfinches (Chloris chloris), one whitewagtail (Motacilla Alba), one owl (Asio otus) and two mallards (Anas platyrhynchos) represent birds from wild habitats. The remaining C. jejuni isolates collected from 47 pigeons (Columba livia), six magpies (Pica pica), six crows (Corvus frugilegus), one pheasant (Phasianus colchicus) and one starling (Sturnus vulgaris) represent birds from urban habitats.
Microbiological analyses and antimicrobial susceptibility tests
The isolates were grown on Columbia blood agar and incubated at 42°C for 48 h in a microaerophilic atmosphere. After preliminary phenotypic characterization, the resultant colonies were confirmed to be thermotolerant C. jejuni using a multiplex PCR, as described by Wang et al. [20], and by a simplex PCR, as described by Di Giannatale et al. [21]. The primers list is shown in Table 1. DNA was extracted using the Maxwell 16 Tissue DNA Purification Kit (Promega Corp., Madison, WI) according to the manufacturer’s instructions. Antimicrobial susceptibility tests on the isolates were performed using the microdilution method to determine the minimum inhibitory concentrations (MICs) of streptomycin (S), ciprofloxacin (cip), tetracycline (Te), gentamicin (G), erythromycin (E) and nalidixic acid (NA), following the harmonised rules for the monitoring and reporting of AMR in Europe (Commission Implementing Decision 2013/652/EC). Briefly, the colonies were grown on Columbia agar for 24 h and then inoculated into Mueller Hinton Broth supplemented with blood (Oxoid, Basingstoke, UK). Then, using the Sensititre® system (Thermo Fisher Scientific, Dardilly, France) the broths were separately dispensed into Eucamp2 microtiter plates (Thermo Fisher Scientific) containing known scalar concentrations of the following antibiotics: S (0.25–16 μg/mL), cip (0.12–16 μg/mL), Te (0.5–64 μg/mL), G (0.12–16 μg/mL) E (1–128 μg/mL) and NA (1–64 μg/mL). After inoculation, the plates were incubated at 42°C in a microaerophilic atmosphere for 24 h and then screened. To evaluate the MICs of the isolates, Swin v3.3 Software (Thermo Fisher Scientific) was used in accordance with the epidemiological cutoff values (ECOFFs) as defined by EUCAST (European Committee on antimicrobial breakpoints) (www.eucast.org) to interpret their antimicrobial susceptibilities. We included the C. jejuni NCTC 11351 reference strain to normalise the MIC tests.
Table 1. List of primers used for PCR.
| Multiplex PCR primers | Sequence (5´-3´) | reference | |
|---|---|---|---|
| C.jejuni | CJF (25 pm) | ACTTCTTTATTGCTTGCTGC | [20] |
| CJR (25 pm) | GCCACAACAAGTAAAGAAGC | [20] | |
| C.coli | CCF (50 pm) | GTAAAACCAAAGCTTATCGTG | [20] |
| CCR (50 pm) | TCCAGCAATGTGTGCAATG | [20] | |
| C.lari | CLF (25 pm) | TAGAGAGATAGCAAAAGAGA | [20] |
| CLR (25 pm) | TACACATAATAATCCCACCC | [20] | |
| C.fetus | CFF (50 pm) | GCAAATATAAATGTAAGCGGAGAG | [20] |
| CFR (50 pm) | TGCAGCGGCCCCACCTAT | [20] | |
| C.upsaliensis | CUF (100 pm) | AATTGAAACTCTTGCTATCC | [20] |
| CUR (100 pm) | TCATACATTTTACCCGAGCT | [20] | |
| Simplex PCR primers | Sequence (5´-3´) | ||
| C.jejuni | P3Fs (50 pm) | GGAAAAACAGGCGTTGTGGGGG | [21] |
| P3Rs (50 pm) | CCGAAGAAGCCATCATCGCACC | [21] |
Identification of antibiotic resistance genes
C. jejuni genome assemblies were searched for the presence of genomic AMR traits. AMR genes were identified in silico using ABRicate v. 0.8 (https://github.com/tseemann/abricate/) and by querying the publicly available Comprehensive Antibiotic Resistance Database [22]http://cge.cbs.dtu.dk/services/ResFinder/. Assemblies were annotated using Prokka v1.13 [23] and gyrA sequences were extracted using the query_pan_genome function in Roary v3.12.0 [24]. gyrA genes were aligned using Uniprot UGENE v1.18.0 [25], from which the gene variants were identified. Only mutations in the quinolone resistance-determining region (QRDR) of gyrA were regarded to be the determinants of resistance, as only these loci have been linked with phenotypic resistance to quinolones. In particular, for gyrA, we analysed the amino acid changes at position 86.
Correlations between phenotypic and genotypic susceptibility to antimicrobials
Correlations between the resistance phenotypes obtained from the Sensititre system and the genetic resistant determinants obtained from the genomic information available at the NRL for Campylobacter for the various monitoring systems were determined for the aforementioned antimicrobials. Specifically, each interpretation for a given phenotypic antibiotic result was manually compared with the presence or absence of the known corresponding resistance gene or with specific mutations, and the percentage correlation between the resistance phenotype and genotype was calculated.
Results
Antimicrobial resistance phenotypes
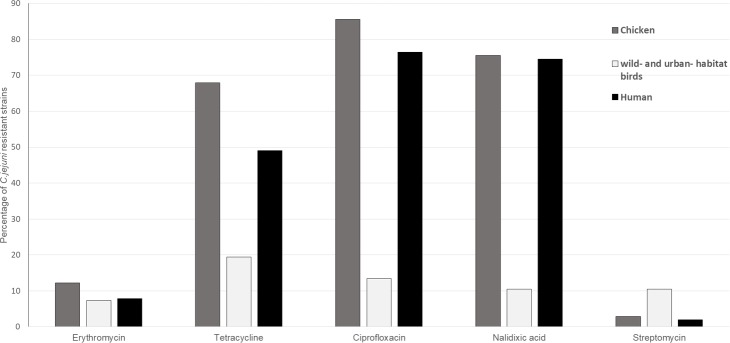
Quinolone resistance was prevalent in C. jejuni from humans, with levels of 76.47% and 74.51% for ciprofloxacin and nalidixic acid, respectively. Ciprofloxacin and nalidixic acid resistance was common in a large portion of the strains from poultry (85.55% and 75.48%, respectively). Tetracycline resistance was also evident in a large percentage of strains, with levels of 49.02% for human isolates and 67.87% for poultry. In contrast, almost all the isolates from humans and poultry were susceptible to aminoglycosides (gentamicin and streptomycin) (Table 2). The antimicrobial test results for the different groups of strains are shown in Fig 1 and Table 2.
Table 2. Percentage of C. jejuni isolates from humans, poultry and birds from wild and urban habitats displaying different antimicrobial susceptibility levels.
| Source | Erythromycin | Gentamycin | Tetracycline | Ciprofloxacin | Nalidixic acid | Streptomycin | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | I | S | R | I | S | R | I | S | R | I | S | R | I | S | R | I | S | |
| Human | 7.84 | 29.41 | 62.75 | 1.96 | 0.00 | 98.04 | 49.02 | 0.00 | 50.98 | 76.47 | 0.00 | 23.53 | 74.51 | 0.00 | 25.49 | 1.96 | 5.88 | 92.16 |
| Chicken—Total | 12.17 | 61.98 | 25.86 | 1.52 | 0.19 | 98.29 | 67.87 | 2.47 | 29.66 | 85.55 | 0.00 | 14.45 | 75.48 | 0.00 | 24.52 | 2.85 | 15.78 | 81.37 |
| Chicken—Animals | 11.07 | 57.86 | 31.07 | 1.07 | 0.36 | 98.57 | 72.86 | 2.50 | 24.64 | 87.86 | 0.00 | 12.14 | 77.86 | 0.00 | 22.14 | 2.14 | 11.79 | 86.07 |
| Chicken—Food | 13.41 | 66.67 | 19.92 | 2.03 | 0.00 | 97.97 | 62.20 | 2.44 | 35.37 | 82.93 | 0.00 | 17.07 | 72.76 | 0.00 | 27.24 | 3.66 | 20.33 | 76.02 |
| Wild and Urban Birds | 7.46 | 82.09 | 10.45 | 2.99 | 0.00 | 97.01 | 19.40 | 1.49 | 79.10 | 13.43 | 0.00 | 86.57 | 10.45 | 0.00 | 89.55 | 10.45 | 7.46 | 82.09 |
R = resistant; S = sensitive; I = intermediate
Fig 1. Percentage of C. jejuni strains isolated from chickens, birds from wild and urban habitats, and from humans showing resistance to antimicrobials (shown on the x-axis).
Conversely, tetracycline resistance was seen more frequently in isolates from the wild and urban habitat birds (19.40%) compared with ciprofloxacin (13.43%), nalidixic acid (10.45%) and streptomycin (10.45%) (Fig 1, Table 2). In the urban habitat, nine pigeon-isolated strains showed tetracycline resistance, six showed ciprofloxacin resistance, five showed nalidixic acid resistance, five showed streptomycin resistance, three showed erythromycin resistance and two showed gentamycin resistance. While one pheasant isolate was resistant to erythromycin, tetracycline, ciprofloxacin and nalidixic acid, one isolate from the six crows was resistant to tetracycline and streptomycin and one isolate from the six magpies was resistant to nalidixic acid and streptomycin. Interestingly, with the birds from wild habitats, one strain from the whitewagtail showed resistance to erythromycin and tetracycline, while the two strains isolated from greenfinches were resistant to tetracycline and ciprofloxacin. In the latter cases, no isolates showed resistance to gentamycin, nalidixic acid and streptomycin.
It is worth noting that the strains isolated from birds inhabiting urban and wild environments, which amounted to 82.09% of the total, showed intermediate levels of susceptibility to erythromycin (Table 2).
The multidrug resistance (MDR) profiles for C. jejuni are shown in Table 3. We identified six C. jejuni-specific antimicrobial resistance profiles. At 47.90% and 17.76%, the TeCipNa MDR category was the most common for the C. jejuni strains isolated from poultry and humans, respectively. Interestingly, isolates from the birds belonging to the wild and urban environments dominated the EGTeCipNaS and ETeCipNaS MDR categories compared with the other isolate groups (poultry and human isolates) (Table 3).
Table 3. Percentage of antimicrobial multi-resistance patterns among C. jejuni from chickens, birds from wild and urban habitats and humans.
| Antibiotic resistance pattern | Chickens–Total (%) | Birds from wild and urban habitats (%) | Humans (%) |
|---|---|---|---|
| EGTeCipNaS (n = 6) | 0.38 | 1.49* | 0.00 |
| ETeCipNaS (n = 5) | 0.90 | 1.49* | 0.00 |
| ETeCipNa (n = 4) | 8.70** | 2.98 | 0.00 |
| TeCipNaS (n = 4) | 1.30 | 1.49¶ | 1.96*** |
| TeCipNa (n = 3) | 47.90≈ | 1.49 | 17.76 |
| CipNaS (n = 3) | 0.00 | 0.00 | 1.96 |
* t-test p<0.001: birds from wild and urban habitats vs. chickens
** t-test p<0.001: chickens vs. birds from wild and urban habitats
¶ t-test p<0.001: birds from wild and urban habitats vs. chickens
*** t-test p<0.001: humans vs. chickens and birds from wild and urban habitats
≈ t-test p<0.001: humans vs. chickens and birds from wild and urban habitats
Detection of resistance genes and mutations, and concordance between resistance phenotypes and genotypes
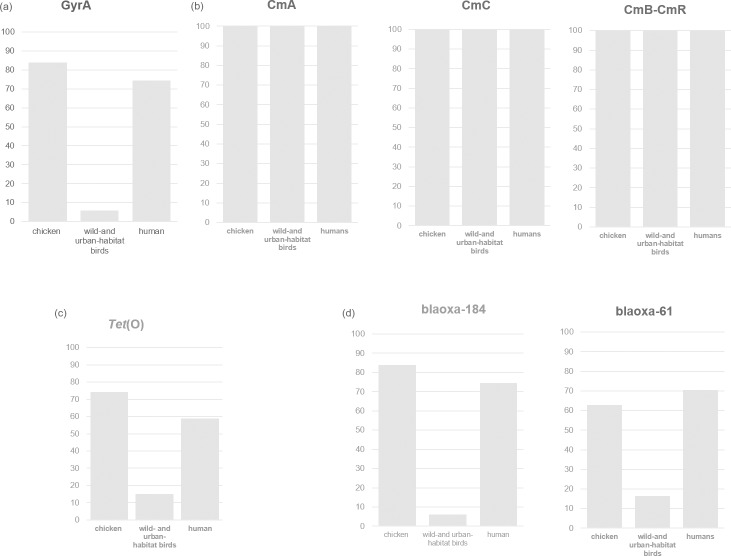
All isolates showing resistance to both ciprofloxacin and nalidixic acid were screened for point mutations within gyrA. With the exception of eight isolates from chickens that carried the A256G point mutation, which produces a T86V substitution, the remaining isolates from chickens, humans and the birds from wild habitats possessed the C257T point mutation, resulting in a T86I substitution in GyrA, a known quinolone resistance mutation (Table 4). The gyrA gene was detected in 83.84% of the isolates from the chickens, in 5.97% of the isolates from birds inhabiting wild and urban areas and in 74.51% of the isolates from humans (Fig 2A). Strong correlations were found for the chicken and human isolates for phenotypic and genotypic resistance, with a high level of concordance (96.22% and 97.43%) for the two resistance rates, respectively. The CmeABC multidrug efflux pump and its CmeR regulator, which together act as a major efflux pump mechanism conferring resistance to a wide range of antimicrobials, were both identified in every strain we analysed (Fig 2B). In the tetracycline-resistant isolates from this study, the tet (O) gene was detected in 74.33% of the chicken isolates, 56.86% of the human isolates and 14.92% of all the isolates from birds (Fig 2C). The correlation percentages between the phenotypes (resistant and susceptible) and genotypes were 93.27% for chickens, 92.30% for birds from wild and urban habitats and 88% for humans (Table 4). Beta-lactam resistance-encoding genes (blaOXA-61 and blaOXA-184) [26] were present at different levels in the analysed species. blaOXA-61 was detected in 70.59% of the human strains, 62.14% of the chicken strains and 17.14% of the strains from the birds from wild and urban habitats (Fig 2D). In contrast, blaOXA-184 was detected in 11.76% of the human strains, 31.56% of the chicken strains and 82.08% of the strains from the birds from wild and urban habitats (Fig 2D). Strong correlations between phenotypic and genotypic resistance were found for fluoroquinolones and tetracycline.
Table 4. Correlations between resistance phenotypes and genotypes among C. jejuni isolates.
| Drug class | drug (s) tested | species | no. of isolates with R phenotype | Presence of resistance genes or mutations corresponding to resistance phenotype (no. of isolates) | Correlation between genotypes and phenotype (%) |
|---|---|---|---|---|---|
| Tetracycline | Te | Chickens | n = 357 | tet (O) (n = 333) | 93.27 |
| Wild birds | n = 13 | tet (O) (n = 12) | 92.30 | ||
| Humans | n = 25 | tet (O) (n = 22) | 88 | ||
| Quinolones, fluoroquinolones | Cip, NA | Chicken | n = 450 | GyrA T86I (n = 433)—GyrA T86V (n = 8) | 96.22 |
| Wild birds | n = 9 | GyrA T86I (n = 4) | 44.44 | ||
| Humans | n = 39 | GyrA T86I (n = 38) | 97.43 |
Fig 2. Percentage of isolates harbouring gyrA and tet (O) genes and the multi-drug resistance-related cmeABC operon, and the percentage of B-lactam genes in the resistant isolates.
Discussion
The increasing trend of drug resistance, particularly MDR, to the major antibiotics currently in use among C. jejuni strains is considered a serious public health problem [27]. A European Union summary report has shown that many worldwide studies have reported on high levels of resistance to ciprofloxacin, nalidixic acid, and tetracycline [28]. Furthermore, a worrying emerging resistance to macrolides was recently observed for Campylobacter [16, 28–29]. In Europe, the rates of fluoroquinolones-resistance in broilers are highly variable, ranging from 1.2% in Norway [30] to 44% in Belgium [31]. An alarming situation was found in Spain where the resistance rate to fluoroquinolones was reported to be 90% [32], while in Poland ciprofloxacin resistance increased from 47.9% during 2005–2008 to 90.2% during 2005–2008 [29]. Several studies from Denmark and Finland have reported that fluoroquinolones and tetracycline resistance rates were significantly higher in travel-associated infections when compared with domestically acquired infections [33–34]. Interestingly, it appears that fluoroquinolone resistance has emerged on poultry farms even in the absence of the above-mentioned antimicrobials [35]. It has been also been suggested that other antimicrobials may select for fluoroquinolone-resistance in Campylobacter [36], but the mechanisms involved are still not completely clarified. For these reasons, continuous monitoring of the resistance rates and investigating the resistance mechanisms is fundamental to combating the potential spread of AMR C. jejuni in humans, as well as across the food chain and in the environment. The present study was undertaken to provide better insight into the dynamics of antibiotic resistance in C. jejuni in Italy by characterising C. jejuni strains from humans, poultry and birds from wild and urban habitats. We also sought to determine whether a correlation exists between the resistance phenotypes and genotypes of the C.jejuni isolates from this study.
Our results show that ciprofloxacin and nalidixic acid resistance was very high in the isolates from humans and poultry (range: 74% to 85%), while 67% of the poultry isolates and approximately half of the human isolates displayed tetracycline resistance. These levels of resistance are consistent with those reported by other recent studies [37]. Similar findings were observed in our previous study of AMR in C. jejuni isolated from broilers, where we noted higher rates of resistance against fluoroquinolones (90%) and similar rates of resistance against tetracycline (64%) [38].
Fluoroquinolones and tetracycline have been used to treat infections in poultry and as growth promoters over the last 50 years [39]. Hence, the high resistance rates to these antimicrobials are likely to be the consequence of their continuous over use [40]. Consistent with this, we observed much lower levels of antibiotic resistance in the isolates obtained from the birds living in urban and wild habitats, which reinforces the argument that the extreme levels of AMR observed in the strains from poultry result from the common use of antibiotics in the farm environment. The MDR profiles of birds from the urban and non-urban habitats suggest that both types of birds could be an important reservoir of MDR C.jejuni strains, and a potential risk population for the spread of resistant bacteria.
Low levels of gentamycin, streptomycin and erythromycin resistance were observed in this study, a finding concordant with previous studies reporting low resistance levels to these antimicrobials in C. jejuni isolated from broiler meat [37–39, 41]. In the present study, 7.84% of the human isolates showed resistance to erythromycin, a higher rate than that identified in similar strains by other authors [42–45] but similar to that reported in a recent study [46]. A recent European Union report stated that the mean European level of erythromycin resistance in C. jejuni was 2.1% in the 21,993 tested isolates [28]. However, a worrying increase in intermediate susceptibility to erythromycin (82.09%) was observed for birds from wild and urban habitats.
MDR, which is defined as resistance to three or more antimicrobial classes [47], has greatly increased worldwide in C. jejuni [39]. In the present study, 47.90% of the chicken strains and 17.76% of the human strains showed MDR phenotypes towards fluoroquinolones and tetracycline, demonstrating the severity of the problem linked to increases in AMR in microorganisms. Although sampling from the birds in the urban habitat was limited, we observed worrying MDR profiles in them, reaching up to five and six drugs. Nevertheless, the infection prevalence rates were statistically significant in the urban habitat-associated birds, suggesting their potential as a vehicle for the transmission of pathogenic C. jejuni and AMR traits to humans.
We screened the quinolone-resistant C. jejuni isolates for the presence of mutations in the QRDR of the gyrA gene. The T86I amino acid substitution was found to be the most common; indeed, it was present in 97.43% and 96.22% of the isolates from humans and poultry, respectively, displaying resistance to ciprofloxacin and nalidixic acid. However, isolates from birds inhabiting the wild and urban study areas harboured a lower percentage of this mutation (44.44%).
We identified another amino acid substitution, T86V, in only eight chicken isolates. Nine poultry isolates were resistant to ciprofloxacin, but they lacked this mutation. These results seem to confirm that quinolone resistance does not depend exclusively on the aforementioned mutations, but can also be attributed to other and/or unknown resistance mechanisms, such as the efflux pump system, as has been also reported in other studies [48–50].
The CmeABC multidrug efflux system, which is the best described multidrug efflux pump to date, plays an important role in antimicrobial resistance. It was present in all the isolates we analysed. The efflux system consists of an outer membrane protein (encoded by CmeC), an internal membrane drug transporter (encoded by CmeB) and a periplasmic protein (encoded by CmeA). Together, these components form a membrane channel that expels toxic substances from the cell [49]. In C. jejuni, the cmeABC operon is negatively regulated by the cmeR repressor, which binds to a 16-base inverted repeat sequence (called the cmeR-Box) located in the promoter region of the cmeABC operon [50–52]. Because a single-nucleotide insertion or deletion in the cmeR-Box has been shown to lead to reduced binding by CmeR, this might in turn increase the expression of cmeABC and enhance the ciprofloxacin resistance level in C. jejuni isolates [53].
The blaOXA-61 gene has been shown to confer resistance to beta-lactams in C. jejuni strains [54]. Over 70% of the human isolates and 80% of the isolates from the wild non-urban habitat birds possessed blaOXA-61 and blaOXA-184 genes, respectively, a finding reported by other authors also [54–55]. However, Campylobacter is intrinsically resistant to beta-lactams; therefore, this class of molecules is not recommended for treating infections caused by this bacterium.
We noted a high correlation between phenotypic resistance to tetracycline and quinolones and the presence of one or more resistance genes or the nucleotide polymorphisms expected to confer resistance to these antimicrobials. For tetracycline, the correlation varied between 88% and 93.27% for the presence of a putative resistance gene and the observed resistance phenotype. A few discrepancies were found with respect to the gyrA mutation and the observed phenotype for the isolates from the birds from wild habitats, which may be explained by the existence of efflux pump mechanisms.
Conclusions
The results of our study suggest that antimicrobial resistance in C. jejuni isolated from humans is correlated with the use of antibiotics in veterinary medicine, and that antimicrobial over use/inappropriate use is an important selective process. Our findings also suggest that multiple resistance patterns to several classes of antibiotics continue to emerge in C. jejuni. Considering the genomic plasticity of Campylobacter and its commensalism with various animal species that are likely to be exposed to different antibiotics, additional resistance mechanisms may continue to evolve in this bacterium. Our data clearly show that antibiotic resistance in Campylobacter is rising. Therefore, AMR monitoring is crucial for designing containment strategies for zoonotic microorganisms like Campylobacter because proper monitoring should help to foresee future AMR spread in animal populations, in humans, and in environmental bacterial populations.
As macrolides are the current treatment choice for campylobacteriosis, the emergence of widespread macrolide resistance remains of primary interest. Knowledge about which genetic resistance elements are present in a bacterial population is crucial for the successful development of new programs of foodborne disease surveillance and control. Phenotypic susceptibility testing, in our opinion, remains fundamental to being able to detect resistance to the principal antimicrobials, even when traditional antibiotic panels can only test a limited number of antibiotics. Additional molecular approaches, such as genomics or proteomics are therefore required to provide new insights into the molecular mechanisms involved in the development of antibiotic resistance in Campylobacter.
To provide information for agricultural production systems and the associated veterinary usage of antimicrobial pharmaceuticals, we also assessed AMR in birds from wild and urban habitats. By addressing the linkage between livestock and wildlife, our study has provided preliminary insight into the potential role of wildlife to act as vectors, reservoirs or amplifiers of antimicrobial resistant microbes. We suggest using birds from wild and urban habitats as key sentinel animals for the surveillance of ecosystem contamination.
Supporting information
(PDF)
Data Availability
We have uploaded our study’s minimal underlying data set as Supporting Information file S1 Table.
Funding Statement
This work was supported by the Italian Ministry of Health, grant number: MSRCTE0717. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, et al. Correction: World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis.PLoSMed. 2015. December 23;12(12):e1001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) (2018). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 16 (12): 5500 10.2903/j.efsa.2018.5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaakoush NO, Deshpande NP, Man SM, Burgos-Portugal JA, Khattak FA, Raftery MJ, et al. Transcriptomic and proteomic analyses reveal key innate immune signatures in the host response to the gastrointestinal pathogen Campylobacter concisus. Infect Immun. 2015;83(2):832–845. 10.1128/IAI.03012-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mughini Gras L, Smid JH, Wagenaar JA, de Boer AG, Havelaar AH, Friesema IH, et al. Risk factors for campylobacteriosis of chicken, ruminant, and environmental origin: a combined case-control and source attribution analysis. PLoS One. 2012;7(8):e42599 Published 2012 Aug 3. 10.1371/journal.pone.0042599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheppard SK, Dallas JF, Strachan NJ, MacRae M, McCarthy ND, Wilson DJ, et al. Campylobacter genotyping to determine the source of human infection. Clin Infect Dis. 2009;48(8):1072–1078. 10.1086/597402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Giannatale E, Garofolo G, Alessiani A, Di Donato G, Candeloro L, Vencia W, et al. Tracing Back Clinical Campylobacter jejuni in the Northwest of Italy and Assessing Their Potential Source. Front Microbiol. 2016. June 13;7:887 10.3389/fmicb.2016.00887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lévesque S, Frost E, Arbeit RD, Michaud S. Multilocus sequence typing of Campylobacter jejuni isolates from humans, chickens, raw milk, and environmental water in Quebec, Canada. J Clin Microbiol. 2008;46(10):3404–3411. 10.1128/JCM.00042-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindmark H, Boqvist S, Ljungström M, Agren P, Björkholm B, Engstrand L. Risk factors for campylobacteriosis: an epidemiological surveillance study of patients and retail poultry. J Clin Microbiol. 2009;47(8):2616–2619. 10.1128/JCM.00826-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitehouse CA, Young S, Li C, Hsu CH, Martin G, Zhao S. Use of whole-genome sequencing for Campylobacter surveillance from NARMS retail poultry in the United States in 2015. Food Microbiol. 2018 Aug;73:122–128. 10.1016/j.fm.2018.01.018 [DOI] [PubMed] [Google Scholar]
- 10.Moore JE, Barton MD, Blair IS, Corcoran D, Dooley JS, Fanning S, et al. The epidemiology of antibiotic resistance in Campylobacter. Microbes Infect. 2006; 8:1955–196. 10.1016/j.micinf.2005.12.030 [DOI] [PubMed] [Google Scholar]
- 11.Avrain L, Humbert F, L'Hospitalier R, Sanders P, Vernozy-Rozand C, Kempf I. Antimicrobial resistance in Campylobacter from broilers: association with production type and antimicrobial use. Vet Microbiol. 2003. October 30;96(3):267–76. 10.1016/j.vetmic.2003.07.001 [DOI] [PubMed] [Google Scholar]
- 12.Janssen R, Krogfelt KA, Cawthraw SA, van Pelt W, Wagenaar JA, Owen RJ. Host-pathogen interactions in Campylobacter infections: the host perspective. Clin. Microbiol 2008; Rev. 21:505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iovine NM. Resistance mechanisms in Campylobacter jejuni. Virulence. 2013;4(3):230–240. 10.4161/viru.23753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaser MJ. Campylobacter and related species In: Mandell G.L., Bennett J.E. & Dolin R. (Eds), Principles and Practice of Infectious Diseases. 4th ed Churchill Livingstone, New York, NY; 1995, p.1948–1956. [Google Scholar]
- 15.Dasti JI, Gross U, Pohl S, Lugert R, Weig M, Schmidt-Ott R. Role of the plasmid-encoded tet(O) gene in tetracycline-resistant clinical isolates of Campylobacter jejuni and Campylobacter coli. J Med Microbiol. 2007. June;56(Pt6):833–7. [DOI] [PubMed] [Google Scholar]
- 16.Luangtongkum T, Jeon B, Han J, Plummer P, Logue CM, Zhang Q. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol. 2009; 4, 189–200. 10.2217/17460913.4.2.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wieczorek K, Osek J. Antimicrobial resistance mechanisms among Campylobacter. BioMed Res. Int. 2013:340605 10.1155/2013/340605 June 17, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.FoodNet—Foodborne Diseases Active Surveillance Network. Centers for Disease Control and Prevention, 2012. (Accessed March 14, 2012, at http://www.cdc.gov/foodnet/.) [Google Scholar]
- 19.Kittl S, Kuhnert P, Hächler H, Korczak BM. Comparison of genotypes and antibiotic resistance of Campylobacter jejuni isolated from humans and slaughtered chickens in Switzerland. J Appl Microbiol 2011; 110:513–20; 10.1111/j.1365-2672.2010.04906.x ; 10.1111/j.1365-2672.2010.04906.x [DOI] [PubMed] [Google Scholar]
- 20.Wang G, Clark CG, Taylor TM, Pucknell C, Barton C, Price L, et al. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp.fetus. J Clin Microbiol. 2002. December;40(12):4744–7. 10.1128/JCM.40.12.4744-4747.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Giannatale E, Garofolo G, Alessiani A, Di Donato G, Candeloro L, Vencia W, et al. Tracing Back Clinical Campylobacter jejuni in the Northwest of Italy and Assessing Their Potential Source. Front Microbiol. 2016. June 13;7:887 10.3389/fmicb.2016.00887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, et al. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017;45(D1):D566–D573. 10.1093/nar/gkw1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seemann T. Prokka: rapid prokaryotic genome annotation Bioinformatics 2014. July 15;30(14):2068–9. 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 24.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MT, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015. November 15;31(22):3691–3. 10.1093/bioinformatics/btv421 Epub 2015 Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okonechnikov K, Golosova O, Fursov M, the UGENE team. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics 2012. 28: 1166–1167. 10.1093/bioinformatics/bts091 [DOI] [PubMed] [Google Scholar]
- 26.Griggs DJ, Peake L, Johnson MM, Ghori S, Mott A, Piddock LJ. Beta-lactamase-mediated beta-lactam resistance in Campylobacter species: prevalence of Cj0299 (bla OXA-61) and evidence for a novel beta-Lactamase in C. jejuni. Antimicrob Agents Chemother. 2009;53(8):3357–3364. 10.1128/AAC.01655-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noormohamed A, Fakhr MK. Prevalence and Antimicrobial Susceptibility of Campylobacter spp. in Oklahoma Conventional and Organic Retail Poultry. Open Microbiol J. 2014;8:130–137. Published 2014 Oct 31. 10.2174/1874285801408010130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.EFSA and ECDC (2018). The European union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2016. EFSA J. 16:e05182 10.2903/j.efsa.2018.5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woźniak A. Fluoroquinolones resistance of Campylobacter jejuni and Campylobacter coli isolated from poultry in 1994–1996 and 2005–2008 in Poland. Bulletin of the Veterinary Institute in Pulawy. 2011;55(1):15–20 [Google Scholar]
- 30.Norström M, Hofshagen M, Stavnes T, Schau J, Lassen J, Kruse H. Antimicrobial resistance in Campylobacter jejuni from humans and broilers in Norway. Epidemiol Infect 2006; 134:127–30; 10.1017/S0950268805004814 ; 10.1017/S0950268805004814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Looveren M, Daube G, De Zutter L, Dumont JM, Lammens C, Wijdooghe M, et al. Antimicrobial susceptibilities of Campylobacter strains isolated from food animals in Belgium. J Antimicrob Chemother 2001; 48:235–40; 10.1093/jac/48.2.235 ; 10.1093/jac/48.2.235 [DOI] [PubMed] [Google Scholar]
- 32.Sáenz Y, Zarazaga M, Lantero M, Gastanares MJ, Baquero F, Torres C. Antibiotic resistance in Campylobacter strains isolated from animals, foods, and humans in Spain in 1997–1998. Antimicrob Agents Chemother. 2000;44(2):267–271. 10.1128/aac.44.2.267-271.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skjøt-Rasmussen L, Ethelberg S, Emborg HD, Agersø Y, Larsen LS, Nordentoft S, et al. Trends in occurrence of antimicrobial resistance in Campylobacter jejuni isolates from broiler chickens, broiler chicken meat, and human domestically acquired cases and travel associated cases in Denmark. Int J Food Microbiol. 2009. May 31; 131(2–3):277–9. 10.1016/j.ijfoodmicro.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 34.Hakanen A, Jousimies-Somer H, Siitonen A, Huovinen P, Kotilainen P. Fluoroquinolone resistance in Campylobacter jejuni isolates in travelers returning to Finland: association of ciprofloxacin resistance to travel destination. Emerg Infect Dis. 2003. February; 9(2):267–70. 10.3201/eid0902.020227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haruna M, Sasaki Y, Murakami M, Ikeda A, Kusukawa M, Tsujiyama Y, et al. Prevalence and antimicro-bial susceptibility of Campylobacter in broiler flocks in Japan. Zoonoses Public Health 2012; 59:241–5; 10.1111/j.1863-2378.2011.01441.x ; 10.1111/j.1863-2378.2011.01441.x [DOI] [PubMed] [Google Scholar]
- 36.Asai T, Harada K, Ishihara K, Kojima A, Sameshima T, Tamura Y, et al. Association of antimicrobial resistance in Campylobacter isolated from food-producing animals with antimicrobial use on farms. Jpn J Infect Dis 2007; 60:290–4; . [PubMed] [Google Scholar]
- 37.Mattheus W, Botteldoorn N, Heylen K, Pochet B, Dierick K. Trend analysis of antimicrobial resistance in Campylobacter jejuni and Campylobacter coli isolated from Belgian pork and poultry meat products using surveillance data of 2004–2009. Foodborne Pathog. 2012; Dis. 9, 465–472. 10.1089/fpd.2011.1042 [DOI] [PubMed] [Google Scholar]
- 38.Marotta F, Garofolo G, Di Donato G, Aprea G, Platone I, Cianciavicchia S, et al. Population Diversity of Campylobacter jejuni in Poultry and Its Dynamic of Contamination in Chicken Meat. Biomed Res Int. 2015;2015:859845 10.1155/2015/859845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giacomelli M., Salata C., Martini M., Montesissa C., Piccirillo A. (2014). Antimicrobial resistance of Campylobacter jejuni and Campylobacter coli from poultry in Italy. Microb. Drug Resist. 20, 181–188. 10.1089/mdr.2013.0110 [DOI] [PubMed] [Google Scholar]
- 40.Elhadidy M, Miller WG, Arguello H, Álvarez-Ordóñez A, Duarte A, Dierick K, et al. Genetic Basis and Clonal Population Structure of Antibiotic Resistance in Campylobacter jejuni Isolated From Broiler Carcasses in Belgium. Front Microbiol. 2018. May 17;9:1014 10.3389/fmicb.2018.01014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Habib I, Miller WG, Uyttendaele M, Houf K, De Zutter L. Clonal population structure and antimicrobial resistance of Campylobacter jejuni in chicken meat from Belgium. Appl Environ Microbiol. 2009;75(13):4264–4272. 10.1128/AEM.00168-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lapierre L, Gatica MA, Riquelme V, Vergara C, Yañez J M, San Martin B. Characterization of antimicrobial susceptibility and its association with virulence genes related to adherence, invasion, and cytotoxicity in Campylobacter jejuni and Campylobacter coli isolates from animals, meat, and humans. Microbial Drug Resist.2016; 22, 432–444. 10.1089/mdr.2015.0055 [DOI] [PubMed] [Google Scholar]
- 43.Cha W, Mosci RE, Wengert SL, Venegas Vargas C, Rust SR, Bartlett PC, et al. Comparing the genetic diversity and antimicrobial resistance profiles of Campylobacter jejuni recovered from cattle and humans. Front. Microbiol. 2017; 8:818 10.3389/fmicb.2017.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riley A, Eshaghi A, Olsha R. Allen VG, Patel SN. Antibiotic susceptibility of clinical isolates of Campylobacter jejuni and Campylobacter coli in Ontario, Canada during 2011–2013. Diagn. Microbiol. Infect. Dis.2015; 83, 292–294. 10.1016/j.diagmicrobio.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 45.Kim JS, Lee MY, Kim SJ, Jeon SE, Cha I, Hong S. High-level ciprofloxacin-resistant Campylobacter jejuni isolates circulating in humans and animals in Incheon, Republic of Korea. Zoonozes Public Health 2016; 63, 545–554. 10.1111/zph.12262 [DOI] [PubMed] [Google Scholar]
- 46.Ghunaim H, Behnke JM, Aigha I, Sharma A, Doiphode SH, Deshmukh A. Analysis of resistance to antimicrobials and presence of virulence/stress response genes in Campylobacter isolates from patients with severe diarrhoea. PLoS ONE 2015; 10:e0119268 10.1371/journal.pone.0119268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwarz S, Silley P, Simjee S, Woodford N, van Duijkeren E, Johnson AP, et al. Review Editorial: assessing the antimicrobial susceptibility of bacteria obtained from animals. J Antimicrob Chemother. 2010. April; 65(4):601–4. 10.1093/jac/dkq037 [DOI] [PubMed] [Google Scholar]
- 48.Bolton D, Patriarchi A, Fox Á, Fanning S. A study of the molecular basis of quinolone and macrolide resistance in a selection of Campylobacter isolates from intensive poultry flocks. Food control. 2013; 30(1), 222–226. [Google Scholar]
- 49.Lin J, Akiba M, Sahin O Zhang Q. CmeR functions as a transcriptional repressor for the multidrug efflux pump CmeABC in Campylobacter jejuni. Antimicrob. Agents Chemother. 2005a; 49, 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin J, Cagliero C, Guo B, Barton Y W, Maurel MC, Payot S, et al. Bile salts modulate expression of the CmeABC multidrug efflux pump in Campylobacter jejuni. J. Bacteriol. 2005b; 187, 7417–7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grinnage-Pulley T, Zhang Q. Genetic Basis and Functional Consequences of Differential Expression of the CmeABC Efflux Pump in Campylobacter jejuni Isolates. PLoS One. 2015; 10(7):e0131534 10.1371/journal.pone.0131534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan M, Sahin O, Lin J, Zhang Q. 2006. Role of the CmeABC efflux pump in the emergence of fluoroquinolone-resistant Campylobacter under selection pressure. J Antimicrob Chemother 58:1154–1159. 10.1093/jac/dkl412 [DOI] [PubMed] [Google Scholar]
- 53.Zhang T, Cheng Y, Luo Q, Lu Q, Dong J, Zhang R, et al. Correlation between gyrA and CmeR Box Polymorphism and Fluoroquinolone Resistance in Campylobacter jejuni Isolates in China. Antimicrob Agents Chemother. 2017. June 27;61(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alfredson DA, Korolik V. 2005. Isolation and expression of a novel molecular class D beta-lactamase, OXA-61, from Campylobacter jejuni. Antimicrob. Agents Chemother., 49 (2005), pp. 2515–2518 10.1128/AAC.49.6.2515-2518.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao S, Tyson GH, Chen Y, Li C, Mukherjee S, Young S, et al. Whole-Genome Sequencing Analysis Accurately Predicts Antimicrobial Resistance Phenotypes in Campylobacter spp. Appl Environ Microbiol. 2015;82(2):459–466. Published 2015 Oct 30. 10.1128/AEM.02873-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
We have uploaded our study’s minimal underlying data set as Supporting Information file S1 Table.