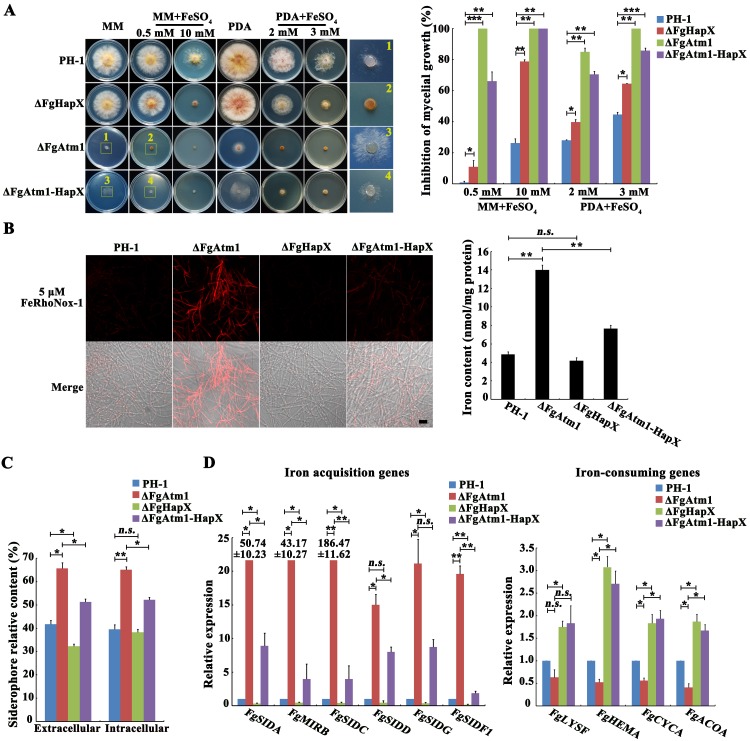
Fig 4. Over-expression of FgHAPX leads to iron accumulation in ΔFgAtm1.
(A) Sensitivity of the wild type, ΔFgAtm1, ΔFgHapX and ΔFgAtm1-HapX to FeSO4. A 5-mm mycelial plug of each strain was inoculated on MM or PDA without or with FeSO4 at the indicated concentration, and then incubated at 25°C for 3 days. Mycelial growth inhibition of each treatment was calculated after 3-day-incubation. Means and standard errors were calculated from three repeats. Significance was measured using unpaired t-test (*p < 0.05, **p < 0.01, ***p < 0.001). (B) Total iron content of the wild type, ΔFgAtm1, ΔFgHapX and ΔFgAtm1-HapX was determined by a laser scanning microscope with 5 μΜ fluorescent iron-binding dye FeRhoNox-1 (left panel) or colorimetric ferrozine-based assay (right panel) after culture in CM at 25°C for 36 hours. Bar = 20 μm. Means and standard errors were calculated from three repeats. Significance was measured using unpaired t-test (*p < 0.05, **p < 0.01). (C) The content of extra- and intracellular siderophores of the wild type, ΔFgAtm1, ΔFgHapX and ΔFgAtm1-HapX determined by the chrome azurol S (CAS) assay. Each strain was cultured in MM lack of Fe2+ for 8 hours after growth in CM for 36 hours. Means and standard errors were calculated from three repeats. Significance was measured using unpaired t-test (n.s. not significant, *p < 0.05, **p < 0.01). (D) Relative transcription levels of the genes involved in iron homeostasis in the wild type, ΔFgAtm1, ΔFgHapX and ΔFgAtm1-HapX cultured in CM for 1 day. The expression level of each gene in each mutant is the relative amount of mRNA compared to the level in the wild type. Mean and standard error of each gene were calculated with results from three repeats. Significance was measured using unpaired t-test (n.s. not significant, *p < 0.05, **p < 0.01).