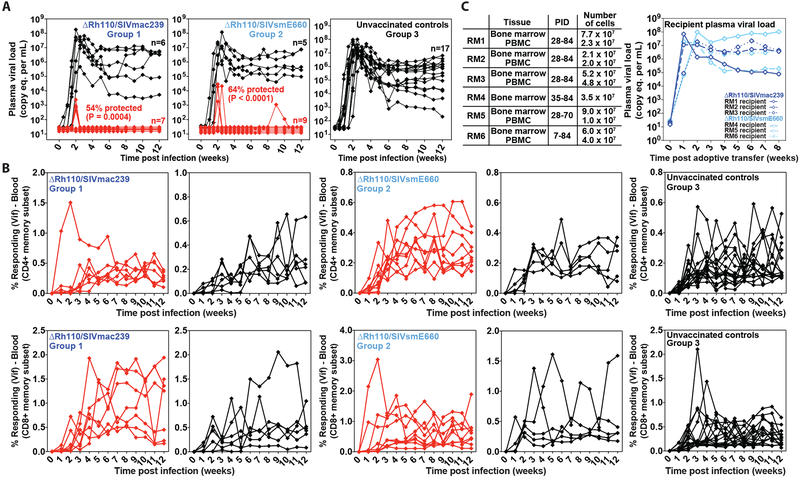
Figure 3: Efficacy of ΔRh110 RhCMV/SIV vectors.
(A,B) Assessment of the outcome of effective challenge by longitudinal analysis of plasma viral load (A) and de novo development of SIV Vif-specific CD4+ (B, top panel) and CD8+ (B, bottom panel) T cell responses. RM were challenged until the onset of any above-threshold SIV Vif-specific T cell response, with the SIV dose administered 2 or 3 weeks prior to this response detection considered the infecting challenge (week 0). RM with sustained viremia were considered not protected (black); RM with no or transient viremia were considered protected (red) (8). The fraction of protected RM in the vaccinated groups (Groups 1 and 2, n = 13 and 14, respectively) were compared to that of the unvaccinated group (Group 3, n = 17) by Barnard’s exact test of binomial proportions, with the P-values shown in (A). (C) BM cells and PBMC were collected and cryopreserved from ΔRh110/SIVmac239/smE660 vaccine-protected RM without any detectable viremia (RM #1, RM #2, RM #3 from Group 1; RM #4, RM #5, RM #6 from Group 2) at the indicated time points post-effective challenge (left panel; PID – post-infection day). Cells were thawed and administered intravenously (left panel) to 6 SIV-naïve RM to assess the presence of replication-competent SIV with the plasma viral dynamics in recipient RM shown (right panel).