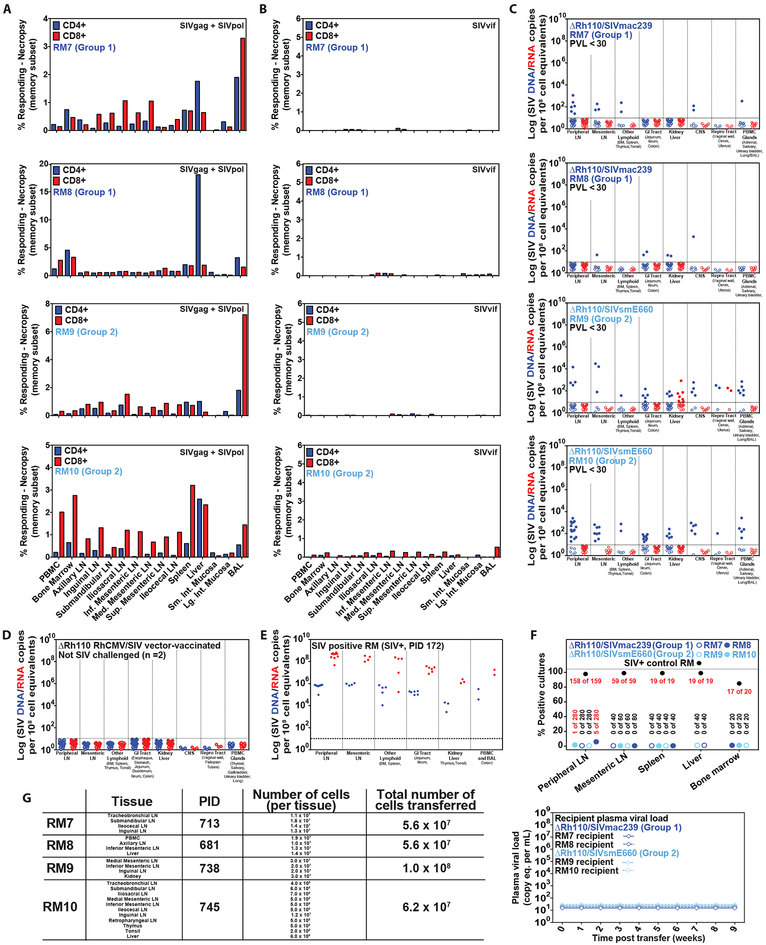
Figure 6: Necropsy analysis of ΔRh110 68–1 RhCMV/SIV vector-protected RM.
(A–C) Analysis of SIV Gag+Pol-specific (A) and SIV Vif-specific (B) CD4+ and CD8+ T cell response frequencies by flow cytometric ICS (using SIVmac239 peptides mixes; see Fig. 1), and tissue-associated SIV DNA and RNA by nested qPCR/RT-PCR (C) in tissues of 4 ΔRh110/SIVmac239/smE660 vector-protected RM (RM #7 and RM #8 from Group 1; RM #9 and RM #10 from Group 2) taken to necropsy at 713 days (RM #7), 681 days (RM #8), 738 days (RM #9) and 745 days (RM #10) post-infection. (D,E) Analysis of tissue-associated SIV DNA and RNA in tissues of 2 ΔRh110 68–1 RhCMV/SIVgag (SIVmac239 sequence insert) vector-vaccinated RM that were taken to necropsy 531 and 763 days post-vaccination without SIV challenge (negative controls; D), and one SIVmac239-infected RM with progressive infection taken to necropsy 172 days post-infection (positive control; E). In C–E, each data point indicates an independent tissue sample of the indicated tissue type and the dotted lines indicate the detection threshold. (F,G) Assessment of residual replication-competent SIV in cell suspensions obtained from the indicated tissue samples by in vitro co-culture analysis (F) and by adoptive transfer of cells into 4 SIV-naïve RM (G).