Abstract
Nanotechnology has been a burgeoning research field which is finding compelling applications in several practical areas of everyday life. It has provided novel, paradigm shifting solutions to medical problems and particularly to cancer. In order to accelerate integration of nanotechnology into cancer research and oncology, the National Cancer Institute (NCI) of the National Institutes of Health (NIH) established the NCI Alliance for Nanotechnology in Cancer program in 2005. This effort brought together scientists representing physical sciences, chemistry, and engineering working at the nanoscale with biologists and clinicians working on cancer to form a uniquely multi-disciplinary cancer nanotechnology research community. The last fourteen years of the program have produced a remarkable body of scientific discovery and demonstrated its utility to the development of practical cancer interventions. This paper takes stock of how the Alliance program influenced melding of disparate research disciplines into the field of nanomedicine and cancer nanotechnology, has been highly productive in the scientific arena, and produced a mechanism of seamless transfer of novel technologies developed in academia to the clinical and commercial space.
Graphical Abstract

Visual Abstract Caption: The National Cancer Institute’s Alliance for Nanotechnology in Cancer has produced numerous technologies dedicated towards novel solutions to cancer. The images depict nanostructures used in novel cancer therapeutics and in vitro or in vivo diagnostics developed by Alliance-funded research.
Introduction
Cancer is the most complex disease known to man. The intense research and translational efforts in this disease space have been paying off with cancer death rates in the U.S. dropping slowly, though consistently, in women by 1.4% and men by 1.8% per annum over the last decade (2007–2016) (Siegel, Miller, & Jemal, 2019). Despite these improvements in death rates and growing number of cancer survivors, it is expected that in 2019, over 1.7M new cancer cases and over 600,000 cancer deaths will occur in the U.S. These statistics continuously drive the quest for new approaches to diagnosis, treatment, and prevention of cancer.
Nanotechnology became of interest to medical researchers towards the end of 20th century. During the Clinton administration, the National Nanotechnology Initiative was established to expand federal funding in this area. Initially, the research in nanotechnology was mostly focused on the development and interrogation of novel materials at nanoscale and engaged predominantly chemists, materials scientists, and physicists. With time it became clear that nanomaterials could facilitate interesting applications, opening new opportunities for medical treatment, among others. Figure 1 shows the evolution of budgets supporting nanotechnology research in different federal funding agencies (“NNI Supplement to the President’s 2019 Budget,” 2018). In the early years, budget levels at Department of Defense and National Science Foundation were high, which was expected due to intense materials research at that time. Since then, their nanotechnology budgets have declined, while the National Institutes of Health budget levels, which increased more slowly, have been maintained at substantial levels.
Figure 1.
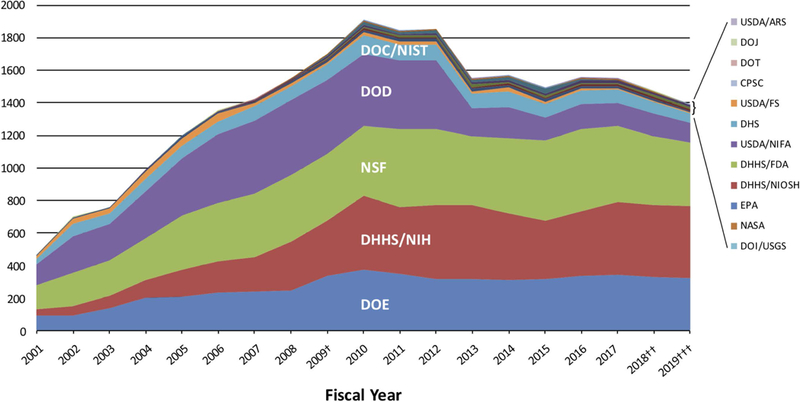
The U.S. government’s funding trends in nanoscale science, engineering, and technology R&D since the inception of the National Nanotechnology Initiative (NNI). The funding levels for nanotechnology by 12 agencies of the U.S. government has maintained high levels since the inception of the NNI due to the 21st Century Nanotechnology Research and Development Act, signed into law in 2003. The agencies that have continued to maintain high levels for the last decade are the National Science Foundation, the Department of Energy, and the National Institutes of Health. (source NNI Supplement to the President’s 2019 Budget – https://www.nano.gov/2019budgetsupplement)
NCI ALLIANCE PROGRAM
In 2005, the National Cancer Institute of the NIH made a bold decision to invest into nanotechnology for cancer. It was apparent from the beginning that for such research to be effective in the medical arena, it needed to involve multi-disciplinary teams consisting of cancer biologists and clinicians working closely with physicists, engineers, and chemists. The Alliance program and its infrastructure have been designed to rapidly advance new discoveries and to transform them into cancer-relevant applications with clinical utility (Chapman et al., 2012; Farrell et al., 2010; Hartshorn et al., 2018; Ptak, Farrell, Panaro, Grodzinski, & Barker, 2010).
Structure and Management
The Alliance funding initiative has fostered the development of basic science to pre-clinical projects in academia, and then moving mature technology platforms forward towards prospective use in the clinic through establishing start-up companies. The program has been funded on 5-year iterations and is currently at the end of its 3rd Phase (i.e., 2005–2020). At the core of the Alliance program are multi-project Centers of Cancer Nanotechnology Excellence (CCNEs), which consist of 2–5 research projects that are further supported by specialized cores. CCNEs were awarded to academic institutions in intense competition, with 6–9 different Centers funded in different Phases of the Alliance program (Figure 2). In addition to CCNEs, single (R01-like) research projects were funded as well along with a number of different training initiatives. The latter involved Path-to-Independence Awards (K99/R00) for promising post-doctoral candidates seeking faculty positions, and T32 Training Centers. NCI also recognized that the development of novel nanomaterials with a wide range of designs requires centralized characterization resources and established an intramural laboratory - the Nanotechnology Characterization Laboratory (NCL). NCL developed a comprehensive assay cascade for nanomaterials characterization and has been working with researchers from academia, industry, and government. NCL has also given back to the field by way of publishing much of the nanomaterials characterization methods they developed (Adiseshaiah, Hall, & McNeil, 2010; Crist et al., 2013; Dobrovolskaia & McNeil, 2016).
Figure 2.
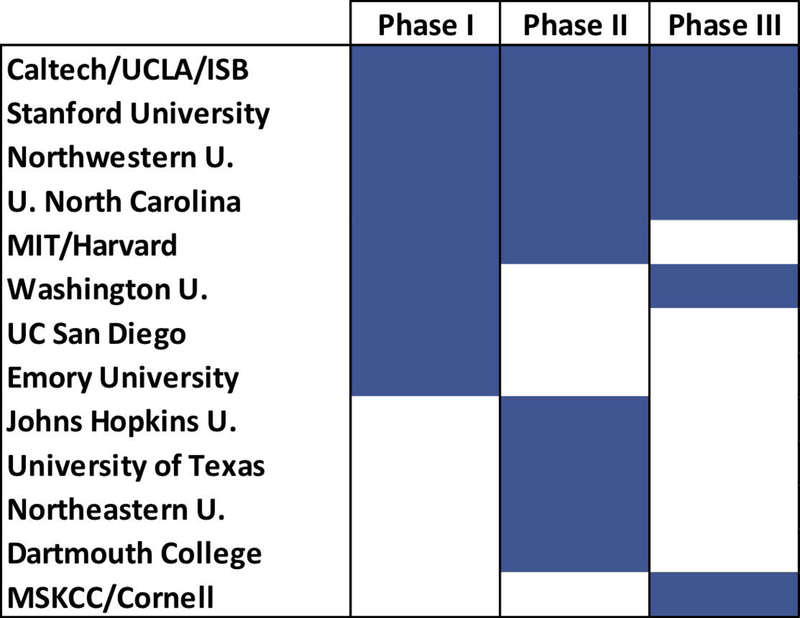
CCNE awards in three different Phases of the Alliance program. Solid blue represents grant awarded during Phase.
NCI Alliance investigators formed a community which is at the fore-front of the rapidly developing cancer nanotechnology field and continues to drive its progress (Farrell, Ptak, Panaro, & Grodzinski, 2011; Nagahara et al., 2010). Several principal investigators (PIs) within the program originate from disciplines that are non-traditional for NIH-sponsored research. These researchers benefit from their partnerships with biologists and clinicians in terms of understanding the needs of contemporary oncology and subsequently direct their research towards the most relevant problems in this area. In turn, they have brought new ideas and innovation to cancer, which may not have occurred without their participation. To assure involvement of oncologists and clinicians in the program to provide an effective guidance on cancer relevant interventions, NCI required multi-investigator leadership of CCNEs and recommended this leadership for smaller grants as well. These requirements led to approximately 30% of the Alliance PIs holding MDs (either MDs or MD/PhDs) on average across all three Phases of the program. This is approximately two times higher than the fraction of investigators with MDs funded through the general NIH NANO study section’s cancer applications, which remains closer to 15% (Figure 3). This points to consistently high clinical involvement among Alliance investigators.
Figure 3.
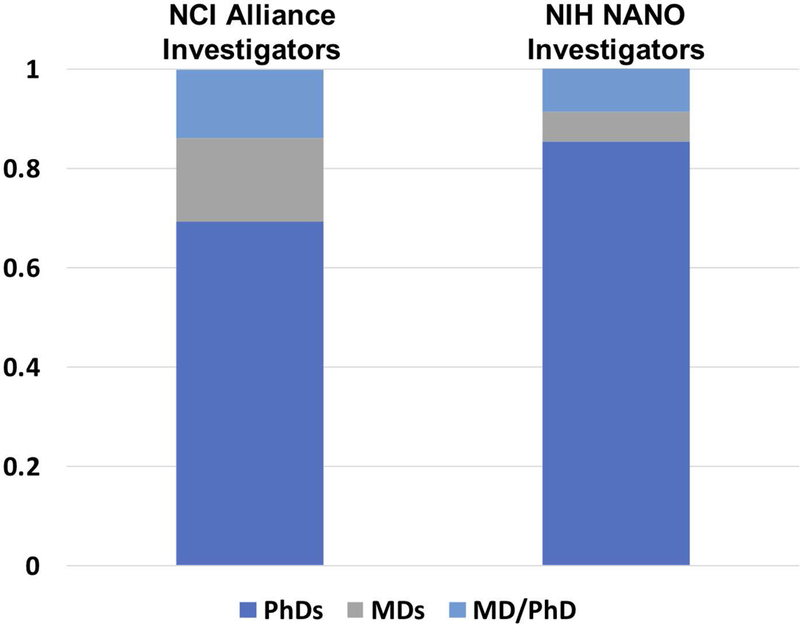
Breakdown of terminal degrees held by grant awardees. In total over the course of all three Phases, Alliance grants tend to include more investigators with clinical degrees than the pool of investigators funded through the general NIH NANO study section’s cancer applications. This includes fraction of funded investigators who are MDs (gray bar) and fraction of funded investigators who hold dual degrees (MDs/PhDs, light blue bar).
As little progress occurs in a vacuum, an active NCI program management structure has ensured frequent and close communication between investigators and the NCI program staff members. This cooperative structure has allowed the NCI to guide, when necessary, the research direction of projects, as well as to promote and facilitate the collaborative efforts among the awardees (Anchordoquy et al., 2017; Grodzinski & Farrell, 2014).
It should be noted that the Alliance program funding constitutes only a portion (~15–20% depending on the program Phase) of the overall NCI funding dedicated to nanotechnology. In parallel to the Alliance-funded grants, the overall number of NCI grant submissions focused on nanotechnology has been growing rapidly during last 10 years, and reached over 700 R01 applications in 2018, which constitutes ~11% of overall R01 submissions (Figure 4). Initially, the majority of nano- R01 submissions were reviewed by the NIH Nanotechnology (NANO) Study Section (formed in 2008). However, as nanotechnology approaches became more mature and were shown to have broader utility, several incoming nano-R01s have started to be assigned to other study sections: Gene and Drug Delivery Systems (GDD), Clinical Molecular Imaging and Probe Development (CMIP), Developmental Therapeutics (DT), and Radiation Therapeutics and Biology (RTB). This indicates growing acceptance of highly innovative nanotechnologies into biomedical research.
Figure 4.
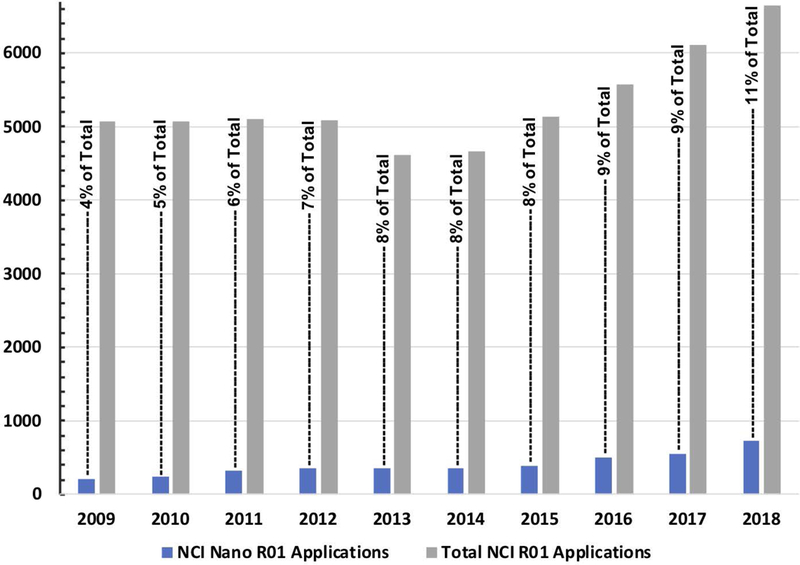
NCI nanotechnology R01 applications versus all NCI R01 applications submitted. The graph highlights the changing level of investigator-initiated R01 applications submitted in the last decade. Applications received and reviewed for nanotechnology (solid blue bars) in comparison with the total number of applications received and reviewed (solid grey bars) displays persistent increase in nanotechnology for cancer being researched by the biomedical research community. The proportion (black text percentiles) of nanotechnology applications to the total submitted highlights this increase. The data was obtained using an internal NIH grant database and contains information on both new and re-submitted grants. Nanotechnology submissions were identified using a search with the NIH RCDC (Research, Condition, and Disease Categorization) term of “nano”.
Scientific Output
Over the last 14 years, Alliance investigators have published over 4,000 peer reviewed research articles that have been collectively cited more than 280,000 times (“Research Published by NCI Alliance,” 2017). This represents substantial scientific dissemination to the biomedical and nanotechnology research communities. Figure 5 represents this cumulative productivity over time. In an earlier stage of the program (in the middle of Phase II), we had attempted to assess to what degree the project teams had coalesced into particular areas of research interests. We have looked at the make-up of publications originating from different investigators within the Alliance program and the evolution of topics covered in these publications (Figure 6). The evolution of topical areas from either biomedical-centric research or nanotechnology development beginnings is apparent. Specifically, we observed a convergence on publication topics centric to cancer, with physical scientists gradually moving their interests from pure nanomaterials and technology development to addressing biology and clinical issues as well as biologists integrating new technologies into their research. This is a strong indication that the multi-disciplinary model of research supported by the Alliance program has been effective and has enabled the convergence of scientists representing diverse disciplines with respect to a single research problem.
Figure 5.
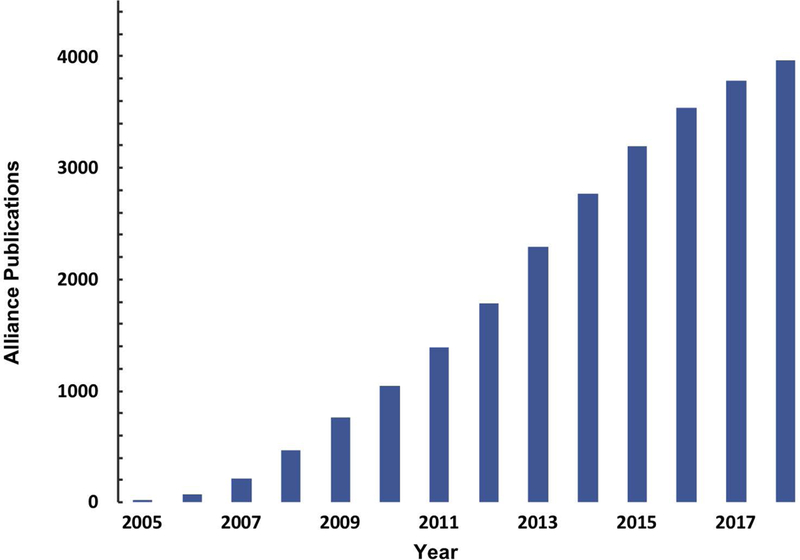
Alliance published scientific output over the course of the program. The cumulative published research by Alliance investigators (dark blue solid bar) between 2005-2018. The data displayed is derived from literature search using NCBI’s PubMed with Alliance-specific grant identifiers for all Alliance publications. Grants included are all Centers of Cancer Nanotechnology Excellence (U54), single research projects (Cancer Nanotechnology Platform Partnership – U01 and Innovative Research in Cancer Nanotechnology – U/R01), Cancer Nanotechnology Training Centers (R25 / T32), and Path-to-Independence Awards (K99/R00) funded under the NCI Alliance program.
Figure 6.
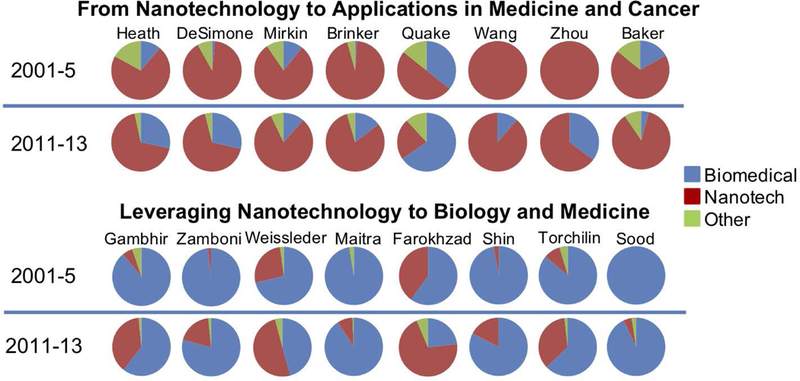
Publication focus of different Alliance investigators. The upper part of the graph represents investigators originating from non-medical fields: physics, chemistry, engineering. As graph shows, prior to their involvement with the Alliance program (2001-2005, 1st, upper row of the graph), they published predominantly in non-medical nanotechnology (indicated as “Nanotech” in red). Once they started to participate via Alliance program funded grants, their focus evolved, and their overall publication portfolio gradually shifted towards medical applications of nanotechnology (visualized by growing blue portion of the pie chart corresponding to “Biomedical” in 2nd upper row). Similarly (lower part of the graph), investigators, who traditionally researched cancer and medicine, began to involve more nanotechnologies in their work as demonstrated by the red portion (“Nanotech”) of the pie chart growing after their participation in the Alliance program.
As discussed in the previous section, the Alliance program was successful in attracting a reasonable number of oncologists and clinicians to its cohort of investigators (Figure 3). We expected that their contributions would impact the translatability of research conducted in the program, which we assessed by two measures of translatability: “Triangle of Medicine” analysis and clinical citation analysis. The Triangle of Medicine and clinical citation analyses were performed using the iTrans software developed at the NIH (Grodzinski, Liu, Hartshorn, Morris, & Russell, 2019). In the Triangle of Medicine analysis, research described in publications is weighted by three categories of biomedical research: human, animal, or molecular/cellular. This kind of analysis provides information on the level of translatability of research (or its maturity towards the clinic), with statistics heavily weighted towards molecular/cellular and animal signifying more basic research, while weighting towards human signifies clinical research. As shown in Figure 7, Alliance research is more heavily weighted towards human research than other nano-grants (reviewed by NANO study section). In addition, the Alliance generated publications had further impact in the clinical space relative to their citation by subsequent clinically-oriented papers authored by both Alliance and non-Alliance investigators, as shown in Figure 8. On average, Alliance research was cited by clinical papers 3.5 times more than research produced by non-Alliance nano-grants.
Figure 7.
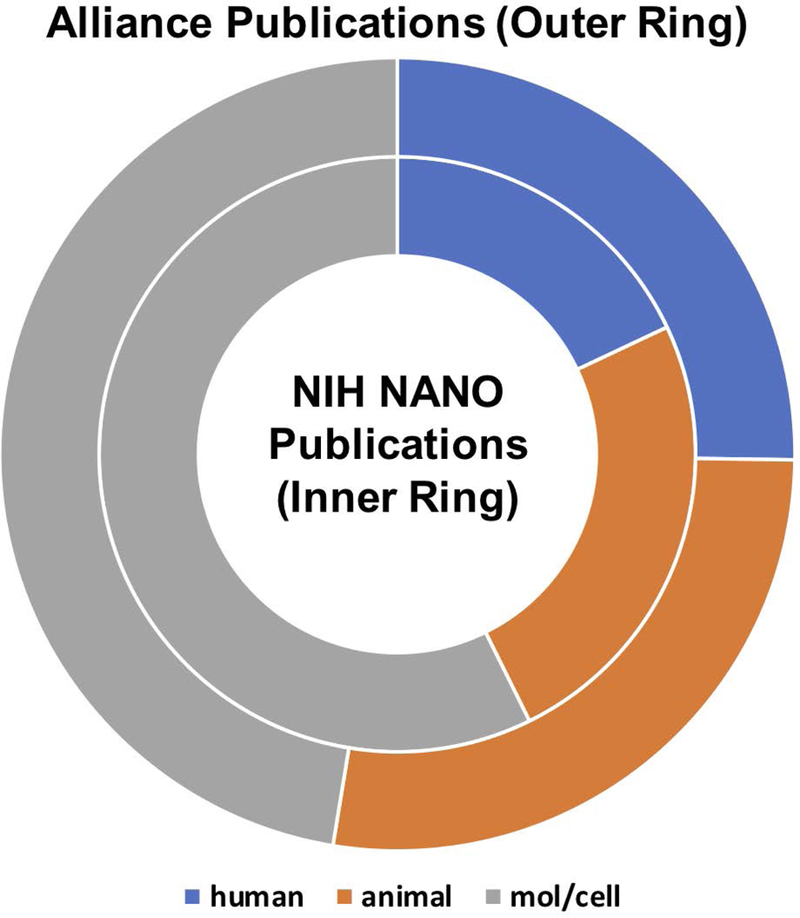
Triangle of Medicine analysis. In this analysis, publications associated with a grant or set of grants are analyzed for the fractions of human, animal, and molecular/cellular research. In this case, the outside ring is representative of Alliance research, with about a quarter of the published research categorized as human (24%, blue section), slightly more than a quarter categorized as animal research (26%, orange section), and slightly less than half of Alliance research categorized as molecular/cellular research (44%, gray section). For comparison, we also analyzed the Triangle of Medicine research composition for all grants funded through the NIH NANO study section, represented in the inside ring. In the case of NIH NANO study section funded grants, only about 16% of published research included a human research component, with 22% animal research and 51% molecular/cellular research.
Figure 8.
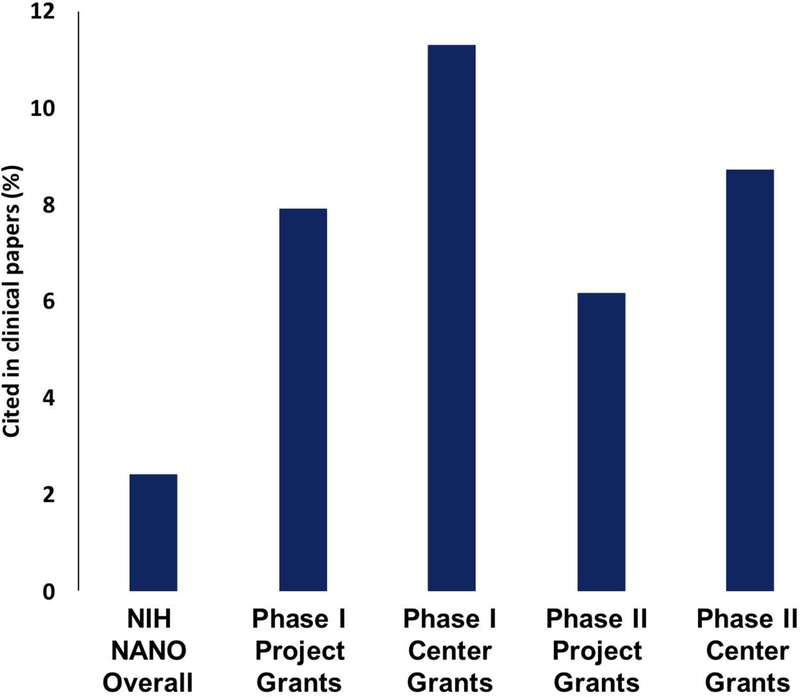
Percent of the Alliance investigator authored publications that are cited by clinical papers – authored by Alliance and/or non-Alliance investigators. This analysis was carried out using iTrans software from the National Institutes of Health – by program Phase and by grant type (Du, Li, Guo, & Tang, 2019). For comparison, this analysis was performed for grants originating from the NIH NANO study section over the period of time corresponding to all three Phases of the Alliance program. The figure displays that both CCNE and smaller Alliance grants tend to be cited more often in subsequent clinical papers when compared to grants originating from the NIH NANO study section.
Nanoparticle and Nano-device Technologies produced by the Alliance
Since the inception of the program, a network of grantees evolved while developing innovative nanotechnologies for prospective medical applications to the biology/oncology driven Alliance program. Proposed projects have increasingly morphed from nanotechnology development to investigations of the processes underlying new therapies (e.g., nanobiology) as well as the patient response to them. Currently, a third of the projects are focused on diagnostic tools for early detection and monitoring of effectiveness of therapy and the remaining two thirds are therapy-centric. But, this breakdown among different cancer applications evolved over the course of the program. The pie charts presented in Figure 9, show the fraction of total projects related to each cancer modality (diagnostic or therapeutic). We observe a strong shift from diagnostic projects (in vitro diagnostics or imaging) in Phase I (Grodzinski, Silver, & Molnar, 2006) to primarily therapeutic projects (e.g., chemotherapy, immunotherapy, gene therapy, radiotherapy, etc) in Phase III. Since the demonstration of clinical utility of the technology is a long-term objective of the program, it was expected that initial project foci would remain on diagnostics due to less complex testing of the devices in in vitro environments. Later on, the therapeutic modalities became more pronounced, particularly with regards to the recent rise of nano-enabled immunotherapy.
Figure 9.
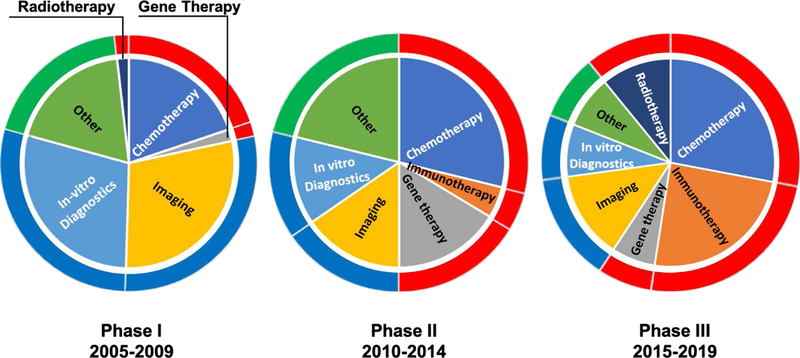
Modalities of Alliance-funded projects over three different Phases of the program. These layered pie charts show the shift in modalities of Alliance-funded projects over the last 15 years. The inner pie chart displays the fraction of total Alliance projects that focused on in vitro diagnostics, imaging, gene therapy, radiotherapy, immunotherapy, chemotherapy, and other during each period. While, the outer ring is color-coded to emphasize the shift from a majority diagnostic (noted in blue) in Phase I to primarily therapeutic (noted in red) projects in Phase III. All other (materials characterization, for example) projects are noted in green.
To give the Reader further insight into various technologies produced by investigators of CCNEs, we catalogued them in Table 1. A similar follow-up table (Table 2) lists representative technologies produced by investigators of smaller grants (R01s and U01s). It is a semi-exhaustive list which allows one the opportunity to appreciate the extent of research and clinical approaches produced by investigators funded by the program. Several of these technologies have been under investigation for many years and matured from early demonstration to that warranting commercialization or even entry into clinical trials. Many of them were also diverse enough in terms of technology platform design to be employed in several cancer applications. For example, spherical nucleic acids (SNAs) were initially developed in Mirkin’s laboratory at Northwestern University to monitor mRNA levels in live cells (Chinen et al., 2015). Later on, they became delivery vehicles used in gene therapy and novel immunotherapies (Li et al., 2018). Kabanov at the University of North Carolina and Torchilin at Northeastern University have been developing micelles for drug delivery applications in cancer for several years. Initially, these works were dedicated to the enhancement of drug loading and biodistribution studies, but gradually evolved to sophisticated nanoparticle designs with variable particle stiffness and shape enabling enhanced nanoparticle penetration into the tumour in Kabanov’s lab (Han et al., 2012; Z. He et al., 2016). Torchilin researched combination therapies and reversal of multidrug resistance (Salzano, Navarro, Trivedi, De Rosa, & Torchilin, 2015). Nanoscale Metal-Organic Frameworks (nMOFs) from Lin’s laboratory at the University of Chicago have become diverse tools for single and combination therapies involving delivery of single and multiple chemotherapeutic drugs and siRNA (C. He, Poon, Chan, Yamada, & Lin, 2016), therapies based on absorption of external radiation and release of oxygen radicals for deep tissue treatment (Lan, Ni, Veroneau, Song, & Lin, 2018; Ni, Lan, Veroneau, et al., 2018), and finally combination of radiation and immunotherapy (K. Lu et al., 2018; Ni, Lan, Chan, et al., 2018). These developed therapeutic platforms cover a broad range of cancer treatment modalities, demonstrating the Alliance therapeutic breadth.
Table 1.
Technologies developed by CCNE awardees. The purpose of this table is to give the Reader an overview of the diversity of projects funded in CCNE grants. We were not able to include all projects – it would make the table too exhaustive. Each CCNE is represented with their most unique projects and also those which progressed to more advanced levels of maturity. The references in the table are not necessarily the most recent ones for a particular technology; they represent the status of work during the time the investigator was holding the grant. The last column in the table lists the company(ies) that licensed the technology for future commercialization and the clinical trial(s) number, if they are is being conducted for the technology.
| Technology and Cancer Application | Investigator/Institution | Publications | Clinical Translation Status (companies and trials) |
|---|---|---|---|
| Diagnostics and Imaging | |||
| Microfluidic-based Integrated Blood Barcode diagnostic devices | James Heath Caltech/UCLA |
(Bailey et al., 2007; Fan et al., 2008b) | Integrated Diagnostics (Biodesix) |
| Monitoring and predicting response to cancer therapies and immunotherapy using Single Cell Barcode Chips | James Heath and Antoni Ribas Caltech/UCLA |
(Ma et al., 2013; Zhou et al., 2018) | Integrated Diagnostics (Biodesix), Isoplexis |
| Devices based on giant magneto-resistance and flow magnetic levitation to detect proteins as well as capture and concentrate circulating cancer cells | Shan Wang and Utkan Demirci Stanford University |
(K. Kim et al., 2018; J.-R. Lee, Magee, Gaster, LaBaer, & Wang, 2013; L.-G. Liang et al., 2017) | MagArray, Levitas |
| Nanoparticles for multi-modal imaging in cancer | Sanjiv Sam Gambhir Stanford University |
(X. He, Gao, Gambhir, & Cheng, 2010; Kircher et al., 2012) | ENDRA Life Sciences |
| Tools for transrectal ultrasound and photoacoustic imaging | Sanjiv Sam Gambhir Stanford University |
(Garai et al., 2013; Willmann et al., 2017) | ENDRA Life Sciences NCT02365883 |
| BarCode assays for in vitro cancer diagnosis | Chad Mirkin Northwestern University |
(Alhasan et al., 2012; Taton, Mirkin, & Letsinger, 2000) | Nanosphere (Luminex) |
| Diagnostic μ-NMR devices based on nanoparticle aggregation for detection of proteins, circulating tumor cells, and microvesicles | Ralph Weissleder MIT/Harvard University |
(Ghazani et al., 2014; Hakho Lee et al., 2009) | T2 Biosystems |
| Spherical nucleic acid (SNAs) nanoconstructs for intracellular imaging | Chad Mirkin and Colby Shad Thaxton Northwestern University |
(Halo et al., 2014; Prigodich et al., 2012) | Aurasense (Exicure) |
| Microfluidic-based screening of DNA methylation for early cancer diagnostics and post-therapy monitoring | Jeff Wang, Stephen Baylin, and James Herman Johns Hopkins University |
(Athamanolap, Shin, & Wang, 2014) | |
| Nanocrystals for multiplexed cancer detection in vitro and in vivo | Shuming Nie, Lily Yang, and Gang Bao Emory University/Georgia Tech |
(A. M. Smith, Dave, Nie, True, & Gao, 2006; Xing, Smith, Agrawal, Ruan, & Nie, 2006) | Ocean Nanotech |
| Carbon nanotube (CNT) X-ray systems to develop instrumentation for radiation therapy and diagnostic medical imaging | Otto Zhou UNC Chapel Hill |
(Hadsell et al., 2013; G. Yang et al., 2011) | XinRay Systems, XinVivo, XinNano Materials, Xintek NCT01773850 |
| Intraoperative sentinel lymph node mapping and radiotherapy using C-dot nanoparticles | Michelle Bradbury and Ulrich Wiesner Memorial Sloan Kettering Cancer Center/Cornell University |
(Bradbury et al., 2016; F. Chen et al., 2018) | Elucida Oncology NCT01266096, NCT02106598 |
| Self-assembling and bio-responsive nanoparticles to probe tumor microenvironment | Jianghong Rao Stanford University |
(G. Liang, Ren, & Rao, 2010; Witney et al., 2015) | |
| Tools to monitor immune cell/cancer cell interactions in novel genetically encoded gas nanovesicles | Mikhail Shapiro and Antoni Ribas ISB/CalTech/UCLA |
(G. J. Lu et al., 2018; Shapiro et al., 2014) | |
| Quantum dots and bacteriophage-inspired nanomaterials for detection of tumor spread | Angela Belcher and Moungi Bawendi MIT/Harvard University |
(Cuneo et al., 2013; Ghosh et al., 2012) | Lumicell, Quantum Dot Corporation |
| Bioresponsive probes for tumor imaging | Martin Pomper Johns Hopkins University |
(Bhang, Gabrielson, Laterra, Fisher, & Pomper, 2011) | Molecular Insight Pharmaceuticals (Progrenics) |
| Novel nanoparticle agents for molecular imaging of cancer | Thomas Meade and Gayle Woloschak Northwestern University |
(Endres, Paunesku, Vogt, Meade, & Woloschak, 2007) | |
| Microfluidic systems for synthesis of PET probes | Hsian-Rong Tseng and Michael Phelps CalTech/UCLA |
(Yanju Wang et al., 2009) | Sofie Biosciences |
| Therapeutics | |||
| Development and translation of targeted polymeric docetaxel nanoparticle therapies for solid tumors | Omid Farokhzad and Robert Langer MIT/Harvard University |
(Behzadi et al., 2017; Farokhzad et al., 2006) | BIND Therapeutics (Pfizer), Blend Therapeutics (Tarveda Therapeutics) Several trials – Table 4 |
| Development and translation of cyclodextrin-containing polymer conjugates of chemotherapeutics and RNAi | Mark Davis CalTech/UCLA |
(Davis, 2009; Davis et al., 2010) | Cerulean Pharma (NewLink Genetics), Several trials – Table 4 Calando Pharmaceuticals NCT00689065 |
| Strategies for crossing physiological barrier using nanoparticles | Mark Davis CalTech/UCLA |
(Clark & Davis, 2015) | |
| PRINT™ nanoparticles for chemotherapy, siRNA, and vaccine delivery | Joseph DeSimone UNC Chapel Hill |
(Mueller, Tian, & DeSimone, 2015; Perry, Herlihy, Napier, & Desimone, 2011) | Liquidia |
| Spherical nucleic acid (SNAs) for gene therapy and design of nanostructure vaccines | Alexander Stegh, Andrew Lee, and Chad Mirkin Northwestern University |
(Jensen et al., 2013; Skakuj et al., 2018; Yue et al., 2018) |
NCT03020017 Exicure, NCT03086278 NCT03684785 |
| Targeted and tumor-penetrating siRNA nanocomplexes for treatment of ovarian cancer | Sangeeta Bhatia and Phillip Sharp MIT/Harvard University |
(Ren et al., 2012) | Alnylam Pharmaceuticals |
| A comprehensive treatment of multiple myeloma through nanoparticle-based tumor microenvironment targeting | Kareem Azab, John DiPersio, and Gregory Lanza Washington University |
(Azab et al., 2012; de la Puente et al., 2018) | Cellatrix, Targeted Therapeutics |
| Deep tissue PDT enabled through the Cerenkov radiation induced therapy (CRIT) | Samuel Achilefu Washington University |
(Kotagiri, Sudlow, Akers, & Achilefu, 2015) | |
| Mucus penetrating nanoparticles for small cell lung cancer | Justin Hanes, Charles Rudin, and Craig Peacock Johns Hopkins University |
(Nance et al., 2012) | Kala Pharmaceuticals |
| Multi-stage nanoparticle vectors for cancer therapeutics | Mauro Ferrari, Paolo Decuzzi, and Gabriel Lopez-Berestein UT/MD Anderson Cancer Center |
(Martinez et al., 2013; Yokoi et al., 2013) | Leonardo Biosystems |
| Mining TCGA data to identify targets for nanotherapies | Anil Sood and Gabriel Lopez-Berestein UT/MD Anderson Cancer Center |
(Shen et al., 2013) | NCT01591356 |
|
Nano-assemblies for ligand-directed imaging and therapy of solid tumors |
Wadih Arap and Renata Pasqualini UT / MD Anderson Cancer Center |
(Hosoya et al., 2016; T. L. Smith et al., 2016) | PhageNova Bio |
| Nanoparticle strategies for siRNA and vaccine delivery | Leaf Huang UNC Chapel Hill |
(Yuhua Wang et al., 2013; Xu et al., 2013) | Qualiber, Inc. |
| Nanoparticle strategies for siRNA and vaccine delivery | Leaf Huang UNC Chapel Hill |
(Yuhua Wang et al., 2013; Xu et al., 2013) | Qualiber, Inc. |
| Nanoparticles for hyperthermia of ovarian and breast cancers | Ian Baker and Jack Hoopes Dartmouth College |
(Stigliano et al., 2013) | |
| Combination therapies to overcome multi-drug resistance (MDR) in cancer | Vladimir Torchilin and Tamara Minko Northeastern University |
(Salzano et al., 2015) | Nemucore Medical Innovations |
| Tumor homing peptide-nanoparticle complexes | Erkki Ruoslahti Sanford Burnham Prebys |
(Sharma et al., 2017; Sugahara et al., 2009) | DrugCendR |
| Nanoparticle strategies for targeting tumor microenvironment | Roger Tsien UC San Diego |
(Crisp et al., 2014) | |
| Silicon oxide porous particles for drug delivery | Michael Sailor and Sadik Esener UC San Diego |
(Anglin, Cheng, Freeman, & Sailor, 2008; Gu, Park, Duong, Ruoslahti, & Sailor, 2010) | Genoptix |
| Targeting angiogenesis using nanoparticle-based imaging and therapy | Gregory Lanza and Samuel Wickline Washington University |
(Winter et al., 2003, 2008) | Kereos |
| Nanoparticles for combination of radio- and immunotherapy | Andrew Wang UNC Chapel Hill |
(Min et al., 2017) | |
| Tools to study cancer biology | |||
| Nanostructured matrices for cancer cell biology interrogations | Milan Mrksich and Bartosz Grzybowski Northwestern University |
(Kandere-Grzybowska et al., 2010; O’Kane & Mrksich, 2017) | SAMDI Tech, Grzybowski Sc. Inventions |
| Nanotechnologies for genome, protein secretion, and biophysical analyses at single cell level | Steve Quake, Luke Lee, and Scott Manalis Stanford University/MIT |
(Bryan et al., 2014; Jianbin Wang, Fan, Behr, & Quake, 2012) | Fluidigm, Travera |
| In vivo spectroscopic quantification of ligand-nanoparticle binding | Brian Pogue and Steven Fiering Dartmouth College |
(Sexton et al., 2013) | |
Table 2.
Technologies developed by R01 and U01 awardees. Similar to Table 2, we were not able to include all projects – it will make the table too exhaustive. We selected the most unique projects and those which progressed to more advanced levels of maturity. The references included in the table are not necessarily the most recent ones for the particular technology; they represent the status of work during the time the investigator was holding the grant. Last column in the table lists the company(ies) which licensed the technology for future commercialization and clinical trial number, if one is being conducted for the technology.
| Cancer Application | Investigator/Institution | Publications | Clinical Translation Status (Companies and Trials) |
|---|---|---|---|
| Diagnostics and imaging | |||
| Devices for circulating tumor cell capture and purification based on thermoresponsive NanoVelcro templates | Hsian-Rong Tseng and Edwin Posadas UCLA/Cedars-Sinai |
(Grossman et al., 2017; Lin et al., 2014) | Cytolumina |
|
Rodent eye as a non-invasive window for
understanding of cancer nanotherapeutics |
Kit Lam UC Davis |
(Goswami et al., 2019) | |
| Imaging of nanotherapeutic drug action | Ralph Weissleder Harvard University |
(Miller & Weissleder, 2017) | VisEn Medical |
| Magnetoresistive sensor platform for parallel cancer marker detection | Mark Porter University of Utah |
(Young, Blackley, Porter, & Granger, 2016) | |
| Nanotechnology platform for pediatric brain cancer imaging and therapy | Miqin Zhang University of Washington |
(Fang & Zhang, 2010) | |
| Therapeutics | |||
| Nanoscale metal-organic frameworks (nMOFs) and coordination polymers (NCPs) for radiodynamic and immunotherapeutic treatment of solid tumors | Wenbin Lin and Ralph Weichselbaum University of Chicago |
(C. He, Duan, et al., 2016; K. Lu et al., 2018) | RiMO Therapeutics, Coordination Pharmaceuticals NCT03444714, NCT03781362 |
| Mesoporous silica nanoparticles for pancreatic cancer treatment | Andre Nel and Huan Meng UCLA |
(J. Lu et al., 2017) | Westwood Bioscience |
| Theranostic nanoparticles for targeted therapy of pancreatic cancer | Lily Yang Emory University |
(Huang et al., 2016) | |
| RNA nanotechnology in cancer therapy | Peixuan Guo Ohio State University |
(Haque & Guo, 2015; Jasinski, Haque, Binzel, & Guo, 2017) | NanoBio Delivery Pharmaceutical |
| Treatment of glioblastoma using chain-like nanoparticles | Efstathios Karathanasis Case Western University |
(Karathanasis & Ghaghada, 2016) | |
| Targeted therapeutics for ovarian cancer and its microenvironment | Gabriel Lopez-Berestein MD Anderson Cancer Center |
(Kanlikilicer et al., 2017) | |
| Overcoming the immune-suppressive tumor microenvironment through in situ vaccination nanotechnology | Nicole Steinmetz UC San Diego/Case Western University |
(Hoopes et al., 2018) | |
| Nanovaccine platforms to combat pancreatic cancer | Balaji Narasimhan Iowa State University |
(Banerjee et al., 2018) | |
| STING-activating polymeric nanovaccines for T cell therapy of melanoma | Jinming Gao UT Southwestern |
(Luo, Samandi, Wang, Chen, & Gao, 2017) | OncoNano Medicine |
| High capacity nanocarriers for cancer chemotherapeutics | Alexander Kabanov UNC Chapel Hill |
(Han et al., 2012; Z. He et al., 2016) | |
| Nanoconjugate based on polymalic acid for brain tumor treatment | Julia Ljubimova Cedars-Sinai |
(Patil et al., 2012) | Arrogene |
| Targeting SYK kinase in B-lineage acute lymphoblastic leukemia (ALL) with CD19-specific C-61 nanoparticles | Fatih Uckun USC |
(Uckun & Qazi, 2014) | |
| Combinatorial nanoplatforms to overcome tumor drug resistance | Mansoor Amiji Northeastern University |
(Ganta & Amiji, 2009; X. Yang et al., 2015) | Nemucore Medical Innovations |
| Toxicity and efficacy of gold nanoparticle photothermal therapy in cancer | Dong Shin Emory University |
(Ali et al., 2017) | |
| Tumor targeted nanobins for the treatment of metastatic breast and ovarian cancer | Thomas O’Halloran Northwestern University |
(S.-M. Lee, O’Halloran, & Nguyen, 2010) | |
| Peptide-directed protocells and virus-like nanoparticle platforms | Cheryl Willman and Jeff Brinker University of New Mexico |
(Ashley et al., 2011) | |
| Preclinical platform for theranostic nanoparticles in pancreatic cancer | Naomi Halas Rice University |
(W. Chen et al., 2014) | Nanospectra Biosciences |
| Photodestruction of ovarian cancer: ErbB3 targeted aptamer-nanoparticle conjugate | Tayyaba Hasan Mass. General Hospital |
(Rai et al., 2010) | Visudyne licensed to Bausch and Lomb |
| Multifunctional nanoparticles in diagnosis and therapy of pancreatic cancer | Paras Prasad SUNY-Buffalo |
(Yong et al., 2009) | Licensed to Nanobiotix |
| Near-infrared fluorescence nanoparticles for targeted optical imaging | Chun Li MD Anderson Cancer Center |
(Zhang et al., 2011) | Carestream Health |
| DNA-linked dendrimer nanoparticle systems for cancer diagnosis and treatment | James Baker University of Michigan |
(Myc et al., 2010) | Avidimer |
| Hybrid nanoparticles in imaging and therapy of prostate cancer | Kattesh Katti University of Missouri |
(Chanda et al., 2010) | Nanoparticle Biochem Inc. |
Similar trends and advancement of technology platforms can be seen in diagnostic technologies funded by the Alliance program. Heath’s laboratory at Caltech developed the DNA Encoded Antibody Library (DEAL) platform, a versatile array technology (Bailey, Kwong, Radu, Witte, & Heath, 2007; Kwong et al., 2009), which after integration with microfluidics was used for multiplex measurements of nucleic acids and proteins. Gradually, this measurement platform evolved into two general classes of devices which provide enabling tools for both clinical oncology and basic cancer biology investigations. The first is the Integrated Blood Barcode Chip (IBBCs), (Fan et al., 2008a; Qin, Vermesh, Shi, & Heath, 2009; Jun Wang et al., 2012), which permits large panels of blood protein biomarkers to be rapidly assayed from just a pinprick of blood. The second platform is the Single Cell Barcode Chip (SCBC). The SCBC permits highly multiplexed assays of secreted, membrane, or cytoplasmic proteins from rare cells and was used to analyse patient samples and profile the immune response to adoptive cell transfer (ACT) cancer immunotherapy (Ma et al., 2013, 2011). The Weissleder laboratory at Harvard University re-used magnetic nanoparticles developed earlier for imaging applications and applied them to the development of new sensing technique based on clustering of these particles upon contact with target proteins. This clustering, in turn, led to more efficient dephasing of nuclear spins of many surrounding water protons in the solutions and changing of spin-spin T2 relaxation time, which could be detected by nuclear magnetic resonance (Ghazani et al., 2014; Hakho Lee, Yoon, Figueiredo, Swirski, & Weissleder, 2009). These sensors were also used in the analysis of circulating tumour cells and exosomes. Wang’s group at Stanford University also developed a sensitive detection platform, but theirs was based on Giant Magnetoresistance (GMR) multilayer material stacks, which undergo a change in resistance upon capturing the target protein labelled with magnetic beads (K. Kim et al., 2018). This sensor platform was also adapted further to capturing circulating tumour cells and circulating tumour DNA (Park et al., 2016; Rizzi et al., 2017). Finally, a joint effort of the Wiesner (Cornell University) and Bradbury (Memorial Sloan Kettering Cancer Center) groups produced C-dots, very small silica-based nanoparticles containing embedded fluorescent dye. C-dots have been used successfully for delineation of tumour margins in the surgery (Bradbury, Pauliah, Zanzonico, Wiesner, & Patel, 2016), but since then are also being adapted for therapeutic applications (F. Chen et al., 2018; S. E. Kim et al., 2016). These are only a few examples which illustrate flexibility and adaptability of nanotechnology platforms in cancer. Further details can be found in Tables 1 and 2. It should be also noted that the majority of nanoparticle platforms developed in the Alliance investigators’ laboratories are non-liposomal ‘next generation’ particles. This is in sharp contrast to the body of particles which had been previously translated to clinical use and received New Drug Approval (NDA) from the FDA, the majority of which were liposomes (Kapoor, Lee, & Tyner, 2017). Figure 10 shows the number of nanoparticle-based clinical trials for liposomes and non-liposomal particles (excluding nab-paclitaxel), with the latter occupying a much smaller fraction of the field. Several of the next generation nanoparticles developed under Alliance funding have entered early clinical trials and hopefully will move forward towards NDAs in the near future.
Figure 10.
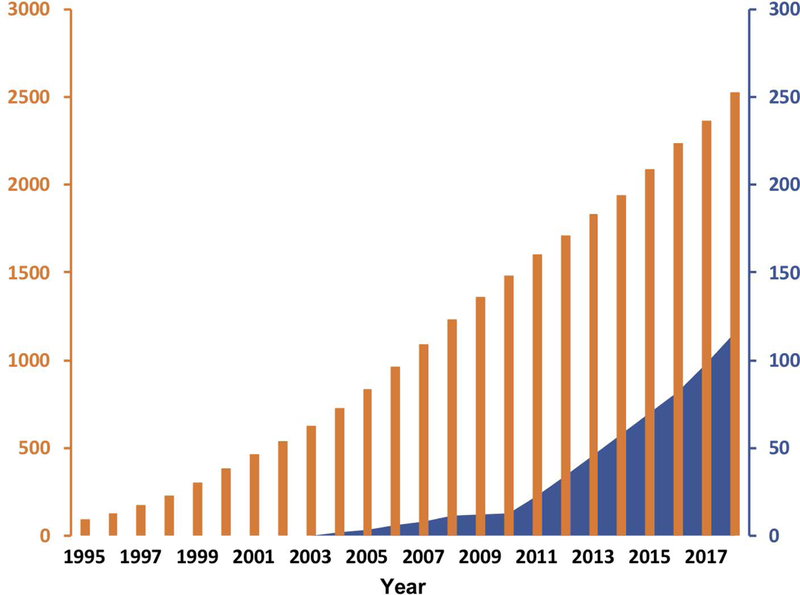
Cumulative cancer clinical trials conducted for liposomal versus non-liposomal nanoparticles over time. This graph displays clinical trials performed for liposomal delivery systems (solid black bars – left axis) since the approval of Doxil in 1995. In comparison, all non-liposomal nanoparticle cancer clinical trials are displayed as solid blue fill, corresponding right axis. The information was derived from clinicaltrials.gov based on the following keyword searches ‘Cancer and – liposomal, Caelyx, Myocet, Lipodox, Onivyde, Depocyt, Daunoxome, Marquibo, Vyxeos, or Mepact’ and for non-liposomal ‘Cancer and nanoparticle or nanotechnology’. The nab-paclitaxel trials that were found were excluded from non-liposomal trials count. The year on the horizontal axis corresponds to the initiation of the trial.
Clinical translation and commercialization of nanotechnology cancer interventions
The initial funding support from the NCI has allowed program participants to secure significant additional research and developmental funds from non-Alliance federal grants, philanthropic sources, industry, and foreign governments in order to further build upon research seeded by the Alliance program. These additional funds allowed for expanding the scope of research performed under Alliance awards, enabled continued work beyond pre-clinical studies, and supported commercialization. Figure 11 represents total dollars leveraged and raised by the four CCNEs that were funded in all three Phases of the Alliance. They are: University of North Carolina CCNE (Nano Approaches to Modulate Host Cell Response for Cancer Therapy), Northwestern University CCNE (Nucleic Acid-Based Nanoconstructs for the Treatment of Cancer), Stanford University CCNE (Center of Cancer Nanotechnology Excellence for Translational Diagnostics), and Caltech-UCLA-ISB CCNE (Nanosystems Biology Cancer Center). The NCI investment in these four CCNEs has cumulatively reached $165 M as of FY2019. Over the course of the same period, these CCNE’s have managed to leverage these NCI funds to obtain other federally funded grants and contracts totalling more than $500 M. When including both leveraged funds obtained and commercial equity raised from companies spun out from these CCNE’s, the total currently exceeds $1.48 B.
Figure 11.
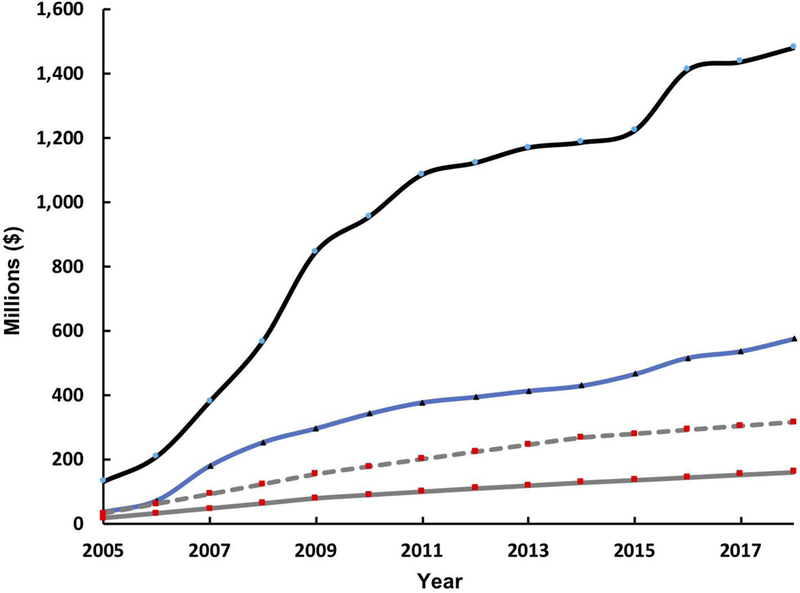
The initial NCI funding and additional funding leveraged by funding to four Alliance CCNEs, which have been funded for all Phases of the program (California Institute of Technology, Northwestern University, Stanford University, and University of North Carolina at Chapel Hill). The graph represents the cumulative investment by NCI to these four CCNEs (gray solid line), the cumulative funds leveraged by these CCNEs from other public funding sources (blue solid line), and the cumulative total of both funds leveraged, and equity invested by companies that commercialized CCNE technologies (black solid line). For additional comparison, the cumulative investment by the NCI to all funded Alliance CCNEs, over all Phases, is also displayed (gray dashed line).
Several of the Alliance investigators have been entrepreneurial and established start-up companies based on licensing of technologies developed in their academic groups. We counted over 125 start-up companies and industrial collaborators associated with the program. Several of these companies have successfully applied for and received SBIR Phase I (i.e., Feasibility and proof of concept stage of funded technology) funding through the NCI SBIR program (Beylin, Chrisman, & Weingarten, 2011; Dickherber, Morris, & Grodzinski, 2015; Narayanan & Weingarten, 2018). Additional funding from private sector was also raised by several of these start-up companies. Of these companies, the majority have successfully filed for and been awarded patents on the underlying technologies. Figure 12 represents patents awarded to companies that span different groups that have been funded by NCI Alliance grants.
Figure 12.
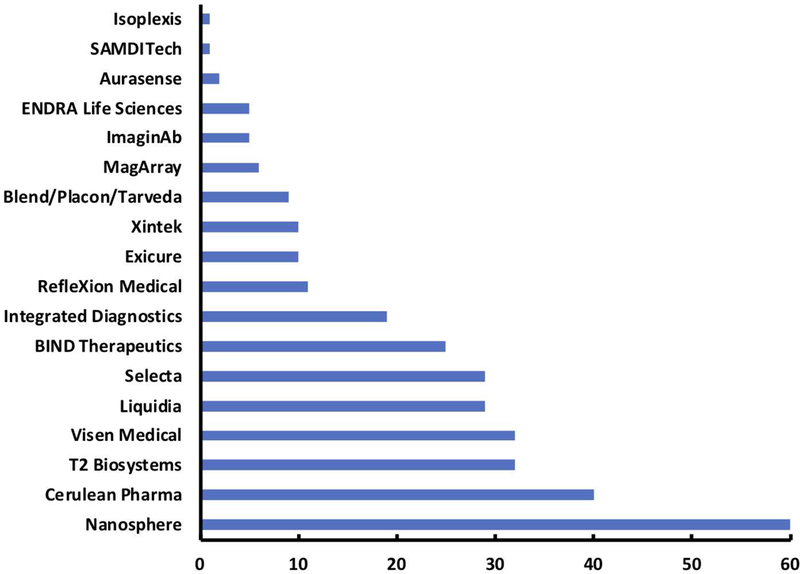
Alliance-related patents. The Alliance program has funded many projects and platform technologies which have ultimately been commercialized and patented by the U.S. Patent and Trademark Office. The graph displays the total number of patents awarded to companies that were either direct spin off companies of Alliance research projects or companies that obtained the intellectual property to their technologies. The company listed do not represent the entire portfolio of companies that came from Alliance investigators and is only a representative sample that covers the most directly related. Patent data was collected using the advanced search feature of Google Patents, where the name of the company was used as the assignee and a filter for US patents only was applied. Of note, not all of the patents listed in the figure came directly from projects funded by the Alliance, as the companies have extensive portfolios and have matured. Yet these companies, and their respective patents relative to Alliance-related companies, are more directly applicable to the original research performed by Alliance investigators who either licensed to existing companies or began the companies themselves.
The importance of this large commercial output, ultimately driving the next-generation nanoscale platforms to the clinic, comes to bear when we analyse the outcomes of this research in the clinical space – that is, the number of clinical trials originated from the Alliance investigators’ research. Several Alliance-associated start-up companies and few universities have thus far entered into 34 clinical trials. A list of Alliance-related clinical trials is shown in Table 3. This table not only shows the high number of clinical trials resulting from technologies developed by the Alliance researchers using Alliance-developed nanoparticles, but also displays the aggressive push into the translation of next-generation, non-liposomal nanoparticles (Gregoriadis, 2016; Shi, Kantoff, Wooster, & Farokhzad, 2017) (e.g., Figure 10).
Table 3.
Table of clinical trials associated with technologies developed by Alliance investigators
| Cancer Indication | Trial Name | Year Started | Phase | Sponsor | Particle Type |
|---|---|---|---|---|---|
| Solid tumor | Study of CRLX101 (formerly named IT-101) in the treatment of advanced solid tumors (NCT00333502) | 2006 | 2 | NewLink Genetics (formerly Cerulean) | Polymer-drug conjugate |
| Solid tumor | Safety study of CALAA-01 to treat solid tumor cancers (NCT00689065) | 2008 | 1 | Calando Pharmaceuticals | Polymeric nanoparticle |
| Metastatic melanoma | Study of gene modified immune cells in patients with advanced melanoma (NCT00910650) | 2009 | 2 | UCLA Jonsson Comprehensive Cancer Center | Diagnostic assay |
| Advanced solid tumors with liver involvement | Evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of intravenous ALN-VSP02 in patients with advanced solid tumors with liver involvement (NCT00882180) | 2009 | 1 | Alnylam Pharmaceuticals | Lipid nanoparticle |
| Advanced solid tumors with liver involvement | Extension study of ALN-VSP02 in cancer patients who have responded to ALN-VSP02 treatment (NCT01158079) | 2010 | 1 | Alnylam Pharmaceuticals | Lipid nanoparticle |
| Non-small cell lung cancer | A Phase 2 study of CRLX101 in patients with advanced non-small cell lung cancer (NCT01380769) | 2011 | 2 | NewLink Genetics (formerly Cerulean) | Polymer-drug conjugate |
| Prostate cancer | Transrectal photoacoustic imaging of the prostate (NCT01380769) | 2011 | NA | Stanford University | Transrectal imaging device |
| Solid tumor, Metastatic cancer | A study of BIND-014 given to patients with advanced or metastatic cancer (NCT01300533) | 2011 | 1 | BIND Therapeutics | Targeted polymeric nanoparticle |
| Metastatic melanoma, Malignant brain tumors | PET imaging of patients with melanoma and malignant brain tumors using an 124I-labeled cRGDY silica nanomolecular particle tracer: A microdosing study (NCT01266096) | 2011 | NA | Memorial Sloan Kettering Cancer Center | Silica nanoparticle (C-dots) |
| Metastatic stomach, gastro-esophageal, or esophageal cancer | Pilot trial of CRLX101 in treatment of patient with advanced or metastatic stomach, gastroesophageal, or esophageal cancer that cannot be removed by surgery (NCT01612546) | 2012 | 2 | NewLink Genetics (formerly Cerulean) | Polymer-drug conjugate |
| Renal cell carcinoma | CRLX101 plus bevacizumab in advanced RCC (NCT01625936) | 2012 | 1 | Cerulean Pharma | Polymer-drug conjugate |
| Ovarian cancer, fallopian tube cancer, and primary peritoneal cancer | CRLX101 in combination with bevacizumab for recurrent ovarian/tubal/peritoneal cancer (NCT01652079) | 2012 | 2 | NewLink Genetics (formerly Cerulean) | Polymer-drug conjugate |
| Rectal cancer | Neoadjuvant chemoradiotherapy with CRLX-101 and capecitabine for rectal cancer (NCT02010567) | 2013 | 2 | NewLink Genetics (formerly Cerulean) | Polymer-drug conjugate |
| Extensive stage or recurrent small cell lung cancer | Topotecan hydrochloride or cyclodextrin-based polymer-camptothecin CRLX101 in treating patients with recurrent small cell lung cancer (NCT01803269) | 2013 | 2 | NewLink Genetics (formerly Cerulean) | Polymer-drug conjugate |
| Prostate cancer, Castration-resistant prostate cancer | A Phase 2 study to determine the safety and efficacy of BIND-014 (Docetaxel nanoparticles for injectable suspension) administered to patients with metastatic castration-resistant prostate cancer (NCT01812746) | 2013 | 2 | BIND Therapeutics | Targeted polymeric nanoparticle |
| Non-small cell lung cancer | A Phase 2 study to determine the safety and efficacy of BIND-014 (Docetaxel nanoparticles for injectable suspension) as second-line therapy to patients with non-small cell lung cancer (NCT01792479) | 2013 | 2 | BIND Therapeutics | Targeted polymeric nanoparticle |
| Breast neoplasms | Comparison of stationary breast tomosynthesis and 2-D digital mammography in patients with known breast lesions (NCT01773850) | 2013 | NA | UNC Lineberger Comprehensive Cancer Center | Carbon nanotubes |
| Metastatic renal cell carcinoma | CRLX101 in combination with bevacizumab for metastatic renal cell carcinoma (mRCC) versus standard of care (SOC) (NCT02187302) | 2014 | 2 | NewLink Genetics (formerly Cerulean) | Polymer-drug conjugate |
| Squamous cell non-small cell lung cancer, KRAS-positive patients with non-small cell lung cancer | A study of BIND-014 (Docetaxel nanoparticles for injectable suspension) as second-line therapy for patients with KRAS-positive or squamous cell non-small cell lung cancer (NCT02283320) | 2014 | 2 | BIND Therapeutics | Targeted polymeric nanoparticles |
| Head and neck melanoma, breast cancer, and colorectal cancers | Targeted silica particles for real-time image-guided intraoperative mapping of nodal metastases (NCT02106598) | 2014 | 2b | Memorial Sloan Kettering Cancer Center | Silica nanoparticles (C-dots) |
| Solid tumors | Alternative dosing for CRLX101 alone, with Avastin and with mFOLFOX6 in advanced solid tumors (NCT02648711) | 2015 | 1 | NewLink Genetics (formerly Cerulean) | Polymer-drug conjugate |
| Ovarian cancer | A Study of CRLX101 in combination with weekly Paclitaxel in patients with recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancer (NCT02389985) | 2015 | 2 | NewLink Genetics (formerly Cerulean) | Polymer-drug conjugate |
| Urothelial carcinoma cholangiocarcinoma, cervical cancer, squamous cell carcinoma of head and neck | A Study of BIND-014 in patients with urothelial carcinoma, cholangiocarcinoma, cervical cancer and squamous cell carcinoma of the head and neck (NCT02479178) | 2015 | 2 | BIND Therapeutics | Targeted polymeric nanoparticle |
| Advanced cancers | Eph2A gene targeting using neutral liposomal small interfering RNA delivery (NCT01591356) | 2015 | 1 | MD Anderson Cancer Center | DOPC nanoliposomes |
| Advanced solid tumor malignancy | Phase 1/2a dose-escalation study of CRLX301 in patients with advanced solid tumors (NCT02380677) | 2015 | 2 | NewLink Genetics (formerly Cerulean) | Polymer-drug conjugate |
| Advanced solid tumors | A Phase 1 study of safety, tolerability, and PK of AZD2811 in patients with advanced solid tumors (NCT02579226) | 2015 | 1 | AstraZeneca | Targeted polymeric nanoparticle |
| Small cell lung carcinoma, non-small-cell lung carcinoma, lung neoplasms, small cell lung cancer, and lung cancer | Trial of CRLX101, a nanoparticle Camptothecin with Olaparib in people with relapsed/refractory small cell lung cancer (NCT02769962) | 2016 | 2 | NewLink Genetics (formerly Cerulean) | Polymer-drug conjugate |
| Gliosarcoma, Recurrent glioblastoma | NU-0129 in treating patients with recurrent glioblastoma or gliosarcoma undergoing surgery (NCT03020017) | 2017 | 1 | Northwestern University | Gold core spherical nucleic acid |
| Healthy volunteers | A study Of AST-008 in healthy subjects (NCT03086278) | 2017 | 1 | Exicure Inc | Gold core spherical nucleic acid |
| Advanced or metastatic: solid tumors, melanoma, head and neck squamous cell carcinoma, cutaneous squamous cell carcinoma, merkel cell carcinoma | Intratumoral AST-008 combined with Pembrolizumab in patients with advanced solid tumors (NCT03684785) | 2018 | 2 | Exicure Inc | Gold core spherical nucleic acid |
| Advanced Tumors | Phase 1 Study of RiMO-301 With Radiation in Advanced Tumors (NCT03444714) | 2018 | 1 | RiMO Therapeutics | Nano metal-organic framework (nMOF) |
| Malignant brain tumors | PET Imaging of Patients With Malignant Brain Tumors Using 89Zr-cRGDY Ultrasmall Silica Particle Tracers: A Phase 1 Microdosing Study (NCT03465618) | 2018 | 1 | Memorial Sloan Kettering Cancer Center | Silica nanoparticles (C-dots) |
| Advanced Tumors | Study of CPI-100 in Patients with Advanced Tumors (NCT03781362) | 2018 | 1 | Coordination Pharmaceuticals | Nanoscale coordination polymer |
| Advanced Tumors | Study of CPI-200 in Patients with Advanced Tumors (NCT03953742) | 2019 | 1 | Coordination Pharmaceuticals | Nanoscale coordination polymer |
Conclusion
The NCI Alliance for Nanotechnology in Cancer program was established in 2005, the same year in which Abraxane was approved by the FDA. Since then, Abraxis Bioscience, the company responsible for the development of Abraxane, was sold to Celgene in 2010; Celgene, in turn, was acquired by Bristol-Myers Squibb in 2019. This scenario reflects the overall dynamism in the field of nanomedicine and cancer nanotechnology. Several iterations and combination trials involving Abraxane have been conducted – clinicaltrials.gov shows 2440 of them. Since 2005, several other nanomedicines were also approved by the US FDA including Marqibo, Onivyde, and Vyxeos – all liposomal formulations of cytotoxic drugs. Nanotherm (particles for hyperthermia treatment of glioblastoma) was recently approved in Europe (Anselmo & Mitragotri, 2016; D’Mello et al., 2017). Recent approval of ONPATTRO™ (patisiran, Alnylam Pharmaceuticals), an RNAi therapeutic agent based on a lipid complex injection for the treatment of the polyneuropathy in amyloidosis (not cancer), signals expansion of the repertoire of therapeutic molecules which can be delivered successfully using nanoparticles.
Despite these successes, nanomedicines, which have reduced life-threatening toxicities of the treatment, have resulted only in modest improvement in the overall survival of patients (Chan, 2017; Chapman et al., 2013; Goldberg et al., 2013; Prabhakar et al., 2013). Further development of nano-therapeutics will undoubtedly emphasize the improvement of patients’ survival. This could be achieved through identifying “niche” applications which are uniquely positioned to benefit from nanotechnology and do not have viable contemporary solutions, expanding the scope of monotherapies to combination therapies delivering two or more synergistic drugs and/or engaging more than one treatment modalities. These strategies can be further facilitated by the implementation of companion diagnostics relying on testing the strength of the Enhanced Permeability and Retention (EPR) effect responsible for nanoparticle accumulation in solid tumours, and resulting in identification of patients for whom nanomedicines may be most effective (Ehlerding, Grodzinski, Cai, & Liu, 2018; Keating et al., 2018; Helen Lee et al., 2017; Miller et al., 2015; Wong, Siah, & Lo, 2018).
The overall landscape in the mature translational nanomedicine space associated with the development of FDA-approved nanodrugs was not directly influenced by the Alliance program, as the entire path from pre-clinical demonstration to the completion of all phases of clinical trials is very lengthy and expensive. Furthermore, many of the early stage developments which resulted in nanomedicines approved by the FDA started prior to the establishment of the Alliance program. However, the Alliance has made a significant mark on establishing next-generation technology platforms suitable for contemporary cancer clinical interventions. This influence has been demonstrated in several areas. The majority of the Alliance nanoparticle platforms belong to next-generation, non-liposomal constructs. As a result, their designs often engage complex materials properties, which can possess therapeutic or diagnostic capabilities, making these constructs more powerful in providing the cancer intervention than simply performing the role of the delivery vehicle (Anselmo & Mitragotri, 2016). The Alliance research has been also opening doors to new areas of cancer applications for nanotechnology and moving beyond nanotechnology-based chemotherapies. As inspection of technologies listed in Tables 1 and 2 show, several therapeutic modalities have employed delivery of pharmaceutical ingredients other than small molecules including biologics, mRNA, and RNAi. The Alliance investigators also ventured into nanotechnology-based immunotherapies and their combination with other treatment modalities. Finally, they made lasting contributions in the development of multi-modal imaging constructs which can be used on different approaches to intra-operative imaging and surgery monitoring.
Many of the innovations emerging from the Alliance work would not occur without the engagement of researchers who are not traditionally NIH grantees: physical scientists, chemists, and engineers. If not for the Alliance program, many of them would not have ended up bringing their talents to focus upon cancer. For example, several of them, who were already prominent in their non-medical fields (chemistry, physics, engineering), were recognized due to their lasting engagement into nanomedicine for their impact to the medical sciences with induction to the National Academy of Medicine. They were Mark Davis, - a chemical engineer from Caltech, Chad Mirkin – a chemist from Northwestern University, Joseph DeSimone – a chemist from the University of North Carolina, and Stephen Quake – a physicist from Stanford University. They also happen to be members of other two National Academies – Engineering, and Sciences, demonstrating the level of prominence of researchers involved in the Alliance program.
The Alliance’s success can be measured not only by the scientific impact, but also by the ability of developing a seamless transition of technologies developed in academia to a further stage of maturity in industry. The Alliance NCI-funded grants supported only academic research. Due to the initiative and commercial acumen of Alliance investigators, multiple start-up companies have been established based on licensing research from their academic groups. These companies raised additional funding, often employed students and post-doctoral fellows from their “parent” academic groups, and successfully moved technologies forward into clinical trials and product development. When the National Academies review the National Nanotechnology Initiative, the Alliance program is featured as a “success story” with multi-faceted path to its success. By all accounts, the program has been considered successful in building a stimulating multi-disciplinary research environment and enriching research seeded by its initial NCI funding through collaborations, innovation, and entrepreneurship.
Acknowledgments
Funding Information
No funding support was used for this review
Contributor Information
Christopher M. Hartshorn, Nanodelivery Systems and Devices Branch, National Cancer Institute, National Institutes of Health, 9609 Medical Center Drive, Rockville, MD 20850, USA.
Luisa M. Russell, Nanodelivery Systems and Devices Branch, National Cancer Institute, National Institutes of Health, 9609 Medical Center Drive, Rockville, MD 20850, USA.
Piotr Grodzinski, Nanodelivery Systems and Devices Branch, National Cancer Institute, National Institutes of Health, 9609 Medical Center Drive, Rockville, MD 20850, USA.
References
- Adiseshaiah PP, Hall JB, & McNeil SE (2010). Nanomaterial standards for efficacy and toxicity assessment. WIRE: Nanomedicine and Nanobiotechnology, 2(1), 99–112. 10.1002/wnan.66 [DOI] [PubMed] [Google Scholar]
- Alhasan AH, Kim DY, Daniel WL, Watson E, Meeks JJ, Thaxton CS, & Mirkin CA (2012). Scanometric microRNA array profiling of prostate cancer markers using spherical nucleic acid-gold nanoparticle conjugates. Analytical Chemistry, 84(9), 4153–4160. 10.1021/ac3004055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MRK, Rahman MA, Wu Y, Han T, Peng X, Mackey MA, … El-Sayed MA (2017). Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice. Proceedings of the National Academy of Sciences, 114(15), E3110–E3118. 10.1073/pnas.1619302114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anchordoquy TJ, Barenholz Y, Boraschi D, Chorny M, Decuzzi P, Dobrovolskaia MA, … Simberg D (2017). Mechanisms and Barriers in Cancer Nanomedicine: Addressing Challenges, Looking for Solutions. ACS Nano, 11(1), 12–18. 10.1021/acsnano.6b08244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglin EJ, Cheng L, Freeman WR, & Sailor MJ (2008). Porous silicon in drug delivery devices and materials. Advanced Drug Delivery Reviews, 60(11), 1266–1277. 10.1016/j.addr.2008.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmo AC, & Mitragotri S (2016). Nanoparticles in the clinic. Bioengineering & Translational Medicine, 1(1), 10–29. 10.1002/btm2.10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley CE, Carnes EC, Phillips GK, Padilla D, Durfee PN, Brown PA, … Brinker CJ (2011). The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nature Materials, 10(5), 389–397. 10.1038/nmat2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athamanolap P, Shin DJ, & Wang T-H (2014). Droplet Array Platform for High-Resolution Melt Analysis of DNA Methylation Density. Journal of Laboratory Automation, 19(3), 304–312. 10.1177/2211068213507923 [DOI] [PubMed] [Google Scholar]
- Azab AK, Quang P, Azab F, Pitsillides C, Thompson B, Chonghaile T, … Ghobrial IM (2012). P-selectin glycoprotein ligand regulates the interaction of multiple myeloma cells with the bone marrow microenvironment. Blood, 119(6), 1468–1478. 10.1182/blood-2011-07-368050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey RC, Kwong GA, Radu CG, Witte ON, & Heath JR (2007). DNA-encoded antibody libraries: a unified platform for multiplexed cell sorting and detection of genes and proteins. Journal of the American Chemical Society, 129(7), 1959–1967. 10.1021/ja065930i [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee K, Kumar S, Ross KA, Gautam S, Poelaert B, Nasser MW, … Jain M (2018). Emerging trends in the immunotherapy of pancreatic cancer. Cancer Letters, 417, 35–46. 10.1016/j.canlet.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi S, Serpooshan V, Tao W, Hamaly MA, Alkawareek MY, Dreaden EC, … Mahmoudi M (2017). Cellular uptake of nanoparticles: journey inside the cell. Chemical Society Reviews, 46(14), 4218–4244. 10.1039/c6cs00636a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beylin D, Chrisman C, & Weingarten M (2011). Granting you success. Bioentrepreneur, 1–4. 10.1038/bioe.2011.5 [DOI] [PubMed] [Google Scholar]
- Bhang HC, Gabrielson KL, Laterra J, Fisher PB, & Pomper MG (2011). Tumor-Specific Imaging through Progression Elevated Gene-3 Promoter-Driven Gene Expression. Nature Medicine, 17(1), 123–129. 10.1038/nm.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury MS, Pauliah M, Zanzonico P, Wiesner U, & Patel S (2016). Intraoperative mapping of sentinel lymph node metastases using a clinically translated ultrasmall silica nanoparticle. WIRE: Nanomedicine and Nanobiotechnology, 8(4), 535–553. 10.1002/wnan.1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan AK, Hecht VC, Shen W, Payer K, Grover WH, & Manalis SR (2014). Measuring single cell mass, volume, and density with dual suspended microchannel resonators. Lab on a Chip, 14(3), 569–576. 10.1039/c3lc51022k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WCW (2017). Nanomedicine 2.0. Accounts of Chemical Research, 50(3), 627–632. 10.1021/acs.accounts.6b00629 [DOI] [PubMed] [Google Scholar]
- Chanda N, Kan P, Watkinson LD, Shukla R, Zambre A, Carmack TL, … Katti KV (2010). Radioactive gold nanoparticles in cancer therapy: therapeutic efficacy studies of GA-198AuNP nanoconstruct in prostate tumor-bearing mice. Nanomedicine: Nanotechnology, Biology, and Medicine, 6(2), 201–209. 10.1016/j.nano.2009.11.001 [DOI] [PubMed] [Google Scholar]
- Chapman S, Dobrovolskaia M, Farahani K, Goodwin A, Joshi A, Lee H, … Yang L (2013). Nanoparticles for cancer imaging: The good, the bad, and the promise. Nano Today, 8(5), 454–460. 10.1016/j.nantod.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S, Panaro NJ, Hinkal GW, Hook SS, Prabhakar U, Ptak K, … Grodzinski P (2012). Kindling translational cancer nanotechnology research. Nanomedicine (London, England), 7(3), 321–325. 10.2217/nnm.12.13 [DOI] [PubMed] [Google Scholar]
- Chen F, Zhang X, Ma K, Madajewski B, Benezra M, Zhang L, … Quinn TP (2018). Melanocortin-1 Receptor-Targeting Ultrasmall Silica Nanoparticles for Dual-Modality Human Melanoma Imaging. ACS Applied Materials & Interfaces, 10(5), 4379–4393. 10.1021/acsami.7b14362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Ayala-Orozco C, Biswal NC, Perez-Torres C, Bartels M, Bardhan R, … Joshi A (2014). Targeting pancreatic cancer with magneto-fluorescent theranostic gold nanoshells. Nanomedicine (London, England), 9(8), 1209–1222. 10.2217/nnm.13.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinen AB, Guan CM, Ferrer JR, Barnaby SN, Merkel TJ, & Mirkin CA (2015). Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence. Chemical Reviews, 115(19), 10530–10574. 10.1021/acs.chemrev.5b00321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AJ, & Davis ME (2015). Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proceedings of the National Academy of Sciences, 112(40), 12486–12491. 10.1073/pnas.1517048112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp JL, Savariar EN, Glasgow HL, Ellies LG, Whitney MA, & Tsien RY (2014). Dual targeting of integrin αvβ3 and matrix metalloproteinase-2 for optical imaging of tumors and chemotherapeutic delivery. Molecular Cancer Therapeutics, 13(6), 1514–1525. 10.1158/1535-7163.MCT-13-1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist RM, Grossman JH, Patri AK, Stern ST, Dobrovolskaia MA, Adiseshaiah PP, … McNeil SE (2013). Common pitfalls in nanotechnology: lessons learned from NCI’s Nanotechnology Characterization Laboratory. Integrative Biology, 5(1), 66 10.1039/c2ib20117h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuneo KC, Mito JK, Javid MP, Ferrer JM, Kim Y, Lee WD, … Kirsch DG (2013). Imaging primary mouse sarcomas after radiation therapy using cathepsin-activatable fluorescent imaging agents. International Journal of Radiation Oncology, Biology, Physics, 86(1), 136–142. 10.1016/j.ijrobp.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ME (2009). Design and development of IT-101, a cyclodextrin-containing polymer conjugate of camptothecin. Advanced Drug Delivery Reviews, 61(13), 1189–1192. 10.1016/j.addr.2009.05.005 [DOI] [PubMed] [Google Scholar]
- Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, … Ribas A (2010). Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature, 464(7291), 1067–1070. 10.1038/nature08956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Puente P, Luderer MJ, Federico C, Jin A, Gilson RC, Egbulefu C, … Azab AK (2018). Enhancing proteasome-inhibitory activity and specificity of bortezomib by CD38 targeted nanoparticles in multiple myeloma. Journal of Controlled Release, 270, 158–176. 10.1016/j.jconrel.2017.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickherber A, Morris SA, & Grodzinski P (2015). NCI investment in nanotechnology: achievements and challenges for the future. WIRE: Nanomedicine and Nanobiotechnology, 7(3), 251–265. 10.1002/wnan.1318 [DOI] [PubMed] [Google Scholar]
- D’Mello SR, Cruz CN, Chen M-L, Kapoor M, Lee SL, & Tyner KM (2017). The evolving landscape of drug products containing nanomaterials in the United States. Nature Nanotechnology, 12(6), 523–529. 10.1038/nnano.2017.67 [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia MA, & McNeil SE (Eds.). (2016). Handbook of Immunological Properties of Engineered Nanomaterials: (Second Edition edition). New Jersey: World Scientific Pub Co Inc. [Google Scholar]
- Du J, Li P, Guo Q, & Tang X (2019). Measuring the knowledge translation and convergence in pharmaceutical innovation by funding-science-technology-innovation linkages analysis. Journal of Informetrics, 13(1), 132–148. 10.1016/j.joi.2018.12.004 [DOI] [Google Scholar]
- Ehlerding EB, Grodzinski P, Cai W, & Liu CH (2018). Big Potential from Small Agents: Nanoparticles for Imaging-Based Companion Diagnostics. ACS Nano, 12(3), 2106–2121. 10.1021/acsnano.7b07252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres PJ, Paunesku T, Vogt S, Meade TJ, & Woloschak GE (2007). DNA−TiO2 Nanoconjugates Labeled with Magnetic Resonance Contrast Agents. Journal of the American Chemical Society, 129(51), 15760–15761. 10.1021/ja0772389 [DOI] [PubMed] [Google Scholar]
- Fan R, Vermesh O, Srivastava A, Yen BKH, Qin L, Ahmad H, … Heath JR (2008a). Integrated barcode chips for rapid, multiplexed analysis of proteins in microliter quantities of blood. Nature Biotechnology, 26(12), 1373–1378. 10.1038/nbt.1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan R, Vermesh O, Srivastava A, Yen BKH, Qin L, Ahmad H, … Heath JR (2008b). Integrated barcode chips for rapid, multiplexed analysis of proteins in microliter quantities of blood. Nature Biotechnology, 26(12), 1373–1378. 10.1038/nbt.1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C, & Zhang M (2010). Nanoparticle-based theragnostics: Integrating diagnostic and therapeutic potentials in nanomedicine. Journal of Controlled Release, 146(1), 2–5. 10.1016/j.jconrel.2010.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, … Langer R (2006). Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proceedings of the National Academy of Sciences, 103(16), 6315–6320. 10.1073/pnas.0601755103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell D, Alper J, Ptak K, Panaro NJ, Grodzinski P, & Barker AD (2010). Recent Advances from the National Cancer Institute Alliance for Nanotechnology in Cancer. ACS Nano, 4(2), 589–594. 10.1021/nn100073g [DOI] [PubMed] [Google Scholar]
- Farrell D, Ptak K, Panaro NJ, & Grodzinski P (2011). Nanotechnology-based cancer therapeutics--promise and challenge--lessons learned through the NCI Alliance for Nanotechnology in Cancer. Pharmaceutical Research, 28(2), 273–278. 10.1007/s11095-010-0214-7 [DOI] [PubMed] [Google Scholar]
- Ganta S, & Amiji M (2009). Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Molecular Pharmaceutics, 6(3), 928–939. 10.1021/mp800240j [DOI] [PubMed] [Google Scholar]
- Garai E, Sensarn S, Zavaleta CL, Van de Sompel D, Loewke NO, Mandella MJ, … Contag CH (2013). High-sensitivity, real-time, ratiometric imaging of surface-enhanced Raman scattering nanoparticles with a clinically translatable Raman endoscope device. Journal of Biomedical Optics, 18(9), 096008. 10.1117/1.JBO.18.9.096008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazani AA, Pectasides M, Sharma A, Castro CM, Mino-Kenudson M, Lee H, … Weissleder R (2014). Molecular characterization of scant lung tumor cells using iron-oxide nanoparticles and micro-nuclear magnetic resonance. Nanomedicine: Nanotechnology, Biology, and Medicine, 10(3), 661–668. 10.1016/j.nano.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Lee Y, Thomas S, Kohli AG, Yun DS, Belcher AM, & Kelly KA (2012). M13-templated magnetic nanoparticles for targeted in vivo imaging of prostate cancer. Nature Nanotechnology, 7(10), 677–682. 10.1038/nnano.2012.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MS, Hook SS, Wang AZ, Bulte JW, Patri AK, Uckun FM, … Mumper RJ (2013). Biotargeted nanomedicines for cancer: six tenets before you begin. Nanomedicine, 8(2), 299–308. 10.2217/nnm.13.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami M, Wang X, Zhang P, Xiao W, Karlen SJ, Li Y, … Pugh EN (2019). Novel window for cancer nanotheranostics: non-invasive ocular assessments of tumor growth and nanotherapeutic treatment efficacy in vivo. Biomedical Optics Express, 10(1), 151–166. 10.1364/BOE.10.000151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriadis G (2016). Liposomes in Drug Delivery: How It All Happened. Pharmaceutics, 8(2), 19 10.3390/pharmaceutics8020019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinski P, & Farrell D (2014). Future Opportunities in Cancer Nanotechnology—NCI Strategic Workshop Report. Cancer Research, 74(5), 1307–1310. 10.1158/0008-5472.CAN-13-2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinski P, Liu CH, Hartshorn CM, Morris SA, & Russell LM (2019). NCI Alliance for Nanotechnology in Cancer – from academic research to clinical interventions. Biomedical Microdevices, 21(2), 32 10.1007/s10544-019-0360-6 [DOI] [PubMed] [Google Scholar]
- Grodzinski P, Silver M, & Molnar LK (2006). Nanotechnology for cancer diagnostics: promises and challenges. Expert Review of Molecular Diagnostics, 6(3), 307–318. 10.1586/14737159.6.3.307 [DOI] [PubMed] [Google Scholar]
- Grossman RL, Abel B, Angiuoli S, Barrett JC, Bassett D, Bramlett K, … Leiman LC (2017). Collaborating to Compete: Blood Profiling Atlas in Cancer (BloodPAC) Consortium. Clinical Pharmacology and Therapeutics, 101(5), 589–592. 10.1002/cpt.666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Park J-H, Duong KH, Ruoslahti E, & Sailor MJ (2010). Magnetic luminescent porous silicon microparticles for localized delivery of molecular drug payloads. Small (Weinheim an Der Bergstrasse, Germany), 6(22), 2546–2552. 10.1002/smll.201000841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadsell M, Zhang J, Laganis P, Sprenger F, Shan J, Zhang L, … Zhou O (2013). A first generation compact microbeam radiation therapy system based on carbon nanotube X-ray technology. Applied Physics Letters, 103(18), 183505 10.1063/1.4826587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halo TL, McMahon KM, Angeloni NL, Xu Y, Wang W, Chinen AB, … Thaxton CS (2014). NanoFlares for the detection, isolation, and culture of live tumor cells from human blood. Proceedings of the National Academy of Sciences, 111(48), 17104–17109. 10.1073/pnas.1418637111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, He Z, Schulz A, Bronich TK, Jordan R, Luxenhofer R, & Kabanov AV (2012). Synergistic combinations of multiple chemotherapeutic agents in high capacity poly(2-oxazoline) micelles. Molecular Pharmaceutics, 9(8), 2302–2313. 10.1021/mp300159u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque F, & Guo P (2015). Overview of methods in RNA nanotechnology: synthesis, purification, and characterization of RNA nanoparticles. Methods in Molecular Biology (Clifton, N.J.), 1297, 1–19. 10.1007/978-1-4939-2562-9_1 [DOI] [PubMed] [Google Scholar]
- Hartshorn CM, Bradbury MS, Lanza GM, Nel AE, Rao J, Wang AZ, … Grodzinski P (2018). Nanotechnology Strategies To Advance Outcomes in Clinical Cancer Care. ACS Nano, 12(1), 24–43. 10.1021/acsnano.7b05108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Duan X, Guo N, Chan C, Poon C, Weichselbaum RR, & Lin W (2016). Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nature Communications, 7, 12499 10.1038/ncomms12499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Poon C, Chan C, Yamada SD, & Lin W (2016). Nanoscale Coordination Polymers Codeliver Chemotherapeutics and siRNAs to Eradicate Tumors of Cisplatin-Resistant Ovarian Cancer. Journal of the American Chemical Society, 138(18), 6010–6019. 10.1021/jacs.6b02486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Gao J, Gambhir SS, & Cheng Z (2010). Near-infrared fluorescent nanoprobes for cancer molecular imaging: status and challenges. Trends in Molecular Medicine, 16(12), 574–583. 10.1016/j.molmed.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Wan X, Schulz A, Bludau H, Dobrovolskaia MA, Stern ST, … Kabanov AV (2016). A high capacity polymeric micelle of paclitaxel: Implication of high dose drug therapy to safety and in vivo anti-cancer activity. Biomaterials, 101, 296–309. 10.1016/j.biomaterials.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes PJ, Wagner RJ, Duval K, Kang K, Gladstone DJ, Moodie KL, … Fiering SN (2018). Treatment of Canine Oral Melanoma with Nanotechnology-Based Immunotherapy and Radiation. Molecular Pharmaceutics, 15(9), 3717–3722. 10.1021/acs.molpharmaceut.8b00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya H, Dobroff AS, Driessen WHP, Cristini V, Brinker LM, Staquicini FI, … Pasqualini R (2016). Integrated nanotechnology platform for tumor-targeted multimodal imaging and therapeutic cargo release. Proceedings of the National Academy of Sciences. 10.1073/pnas.1525796113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Li Y, Orza A, Lu Q, Guo P, Wang L, … Mao H (2016). Magnetic Nanoparticle Facilitated Drug Delivery for Cancer Therapy with Targeted and Image-Guided Approaches. Advanced Functional Materials, 26(22), 3818–3836. 10.1002/adfm.201504185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski D, Haque F, Binzel DW, & Guo P (2017). Advancement of the Emerging Field of RNA Nanotechnology. ACS Nano, 11(2), 1142–1164. 10.1021/acsnano.6b05737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, … Stegh AH (2013). Spherical Nucleic Acid Nanoparticle Conjugates as an RNAi-Based Therapy for Glioblastoma. Science Translational Medicine, 5(209), 209ra152–209ra152. 10.1126/scitranslmed.3006839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandere-Grzybowska K, Soh S, Mahmud G, Komarova Y, Pilans D, & Grzybowski BA (2010). Short-term molecular polarization of cells on symmetric and asymmetric micropatterns. Soft Matter, 6(14), 3257–3268. 10.1039/B922647H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanlikilicer P, Ozpolat B, Aslan B, Bayraktar R, Gurbuz N, Rodriguez-Aguayo C, … Lopez-Berestein G (2017). Therapeutic Targeting of AXL Receptor Tyrosine Kinase Inhibits Tumor Growth and Intraperitoneal Metastasis in Ovarian Cancer Models. Molecular Therapy - Nucleic Acids, 9, 251–262. 10.1016/j.omtn.2017.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Lee SL, & Tyner KM (2017). Liposomal Drug Product Development and Quality: Current US Experience and Perspective. The AAPS Journal, 19(3), 632–641. 10.1208/s12248-017-0049-9 [DOI] [PubMed] [Google Scholar]
- Karathanasis E, & Ghaghada KB (2016). Crossing the barrier: treatment of brain tumors using nanochain particles. WIRE: Nanomedicine and Nanobiotechnology, 8(5), 678–695. 10.1002/wnan.1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating SM, Taylor DL, Plant AL, Litwack ED, Kuhn P, Greenspan EJ, … Kuida K (2018). Opportunities and Challenges in Implementation of Multiparameter Single Cell Analysis Platforms for Clinical Translation. Clinical and Translational Science, 11(3), 267–276. 10.1111/cts.12536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Hall DA, Yao C, Lee J-R, Ooi CC, Bechstein DJB, … Wang SX (2018). Magnetoresistive biosensors with on-chip pulsed excitation and magnetic correlated double sampling. Scientific Reports, 8(1), 16493 10.1038/s41598-018-34720-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Zhang L, Ma K, Riegman M, Chen F, Ingold I, … Overholtzer M (2016). Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nature Nanotechnology, 11(11), 977–985. 10.1038/nnano.2016.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher MF, de la Zerda A, Jokerst JV, Zavaleta CL, Kempen PJ, Mittra E, … Gambhir SS (2012). A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nature Medicine, 18(5), 829–834. 10.1038/nm.2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotagiri N, Sudlow GP, Akers WJ, & Achilefu S (2015). Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nature Nanotechnology, 10(4), 370–379. 10.1038/nnano.2015.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong GA, Radu CG, Hwang K, Shu CJ, Ma C, Koya RC, … Heath JR (2009). Modular nucleic acid assembled p/MHC microarrays for multiplexed sorting of antigen-specific T cells. Journal of the American Chemical Society, 131(28), 9695–9703. 10.1021/ja9006707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan G, Ni K, Veroneau SS, Song Y, & Lin W (2018). Nanoscale Metal-Organic Layers for Radiotherapy-Radiodynamic Therapy. Journal of the American Chemical Society. 10.1021/jacs.8b11593 [DOI] [PubMed] [Google Scholar]
- Lee Hakho, Yoon T-J, Figueiredo J-L, Swirski FK, & Weissleder R (2009). Rapid detection and profiling of cancer cells in fine-needle aspirates. Proceedings of the National Academy of Sciences, 106(30), 12459–12464. 10.1073/pnas.0902365106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Helen, Shields AF, Siegel BA, Miller KD, Krop I, Ma CX, … Hendriks BS (2017). 64Cu-MM-302 Positron Emission Tomography Quantifies Variability of Enhanced Permeability and Retention of Nanoparticles in Relation to Treatment Response in Patients with Metastatic Breast Cancer. Clinical Cancer Research, 23(15), 4190–4202. 10.1158/1078-0432.CCR-16-3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-R, Magee DM, Gaster RS, LaBaer J, & Wang SX (2013). Emerging protein array technologies for proteomics. Expert Review of Proteomics, 10(1), 65–75. 10.1586/epr.12.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-M, O’Halloran TV, & Nguyen ST (2010). Polymer-caged nanobins for synergistic cisplatin-doxorubicin combination chemotherapy. Journal of the American Chemical Society, 132(48), 17130–17138. 10.1021/ja107333g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang B, Lu X, Tan X, Jia F, Xiao Y, … Mirkin CA (2018). Molecular spherical nucleic acids. Proceedings of the National Academy of Sciences, 115(17), 4340–4344. 10.1073/pnas.1801836115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Ren H, & Rao J (2010). A biocompatible condensation reaction for controlled assembly of nanostructures in living cells. Nature Chemistry, 2(1), 54–60. 10.1038/nchem.480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L-G, Sheng Y-F, Zhou S, Inci F, Li L, Demirci U, & Wang S (2017). An Integrated Double-Filtration Microfluidic Device for Detection of Extracellular Vesicles from Urine for Bladder Cancer Diagnosis. Methods in Molecular Biology (Clifton, N.J.), 1660, 355–364. 10.1007/978-1-4939-7253-1_29 [DOI] [PubMed] [Google Scholar]
- Lin M, Chen J-F, Lu Y-T, Zhang Y, Song J, Hou S, … Tseng H-R (2014). Nanostructure embedded microchips for detection, isolation, and characterization of circulating tumor cells. Accounts of Chemical Research, 47(10), 2941–2950. 10.1021/ar5001617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu GJ, Farhadi A, Szablowski JO, Lee-Gosselin A, Barnes SR, Lakshmanan A, … Shapiro MG (2018). Acoustically modulated magnetic resonance imaging of gas-filled protein nanostructures. Nature Materials, 17(5), 456 10.1038/s41563-018-0023-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Liu X, Liao Y-P, Salazar F, Sun B, Jiang W, … Nel AE (2017). Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nature Communications, 8(1), 1811 10.1038/s41467-017-01651-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, He C, Guo N, Chan C, Ni K, Lan G, … Lin W (2018). Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nature Biomedical Engineering, 2(8), 600 10.1038/s41551-018-0203-4 [DOI] [PubMed] [Google Scholar]
- Luo M, Samandi LZ, Wang Z, Chen ZJ, & Gao J (2017). Synthetic nanovaccines for immunotherapy. Journal of Controlled Release, 263, 200–210. 10.1016/j.jconrel.2017.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Cheung AF, Chodon T, Koya RC, Wu Z, Ng C, … Heath JR (2013). Multifunctional T-cell analyses to study response and progression in adoptive cell transfer immunotherapy. Cancer Discovery, 3(4), 418–429. 10.1158/2159-8290.CD-12-0383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Fan R, Ahmad H, Shi Q, Comin-Anduix B, Chodon T, … Heath JR (2011). A clinical microchip for evaluation of single immune cells reveals high functional heterogeneity in phenotypically similar T cells. Nature Medicine, 17(6), 738–743. 10.1038/nm.2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JO, Chiappini C, Ziemys A, Faust AM, Kojic M, Liu X, … Tasciotti E (2013). Engineering multi-stage nanovectors for controlled degradation and tunable release kinetics. Biomaterials, 34(33), 8469–8477. 10.1016/j.biomaterials.2013.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Gadde S, Pfirschke C, Engblom C, Sprachman MM, Kohler RH, … Weissleder R (2015). Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Science Translational Medicine, 7(314), 314ra183. 10.1126/scitranslmed.aac6522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, & Weissleder R (2017). Imaging of anticancer drug action in single cells. Nature Reviews. Cancer, 17(7), 399–414. 10.1038/nrc.2017.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Y, Roche KC, Tian S, Eblan MJ, McKinnon KP, Caster JM, … Wang AZ (2017). Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nature Nanotechnology, 12(9), 877–882. 10.1038/nnano.2017.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Tian S, & DeSimone JM (2015). Rapid and Persistent Delivery of Antigen by Lymph Node Targeting PRINT Nanoparticle Vaccine Carrier To Promote Humoral Immunity. Molecular Pharmaceutics, 12(5), 1356–1365. 10.1021/mp500589c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myc A, Kukowska-Latallo J, Cao P, Swanson B, Battista J, Dunham T, & Baker JR (2010). Targeting the efficacy of a dendrimer-based nanotherapeutic in heterogeneous xenograft tumors in vivo. Anti-Cancer Drugs, 21(2), 186–192. 10.1097/CAD.0b013e328334560f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara LA, Lee JSH, Molnar LK, Panaro NJ, Farrell D, Ptak K, … Grodzinski P (2010). Strategic workshops on cancer nanotechnology. Cancer Research, 70(11), 4265–4268. 10.1158/0008-5472.CAN-09-3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance EA, Woodworth GF, Sailor KA, Shih T-Y, Xu Q, Swaminathan G, … Hanes J (2012). A Dense Poly(Ethylene Glycol) Coating Improves Penetration of Large Polymeric Nanoparticles Within Brain Tissue. Science Translational Medicine, 4(149), 149ra119–149ra119. 10.1126/scitranslmed.3003594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan D, & Weingarten M (2018). Chapter 9 - An Introduction to the National Institutes of Health SBIR/STTR Programs In Behrns KE, Gingles B, & Sarr MG(Eds.), Medical Innovation (pp. 87–100). 10.1016/B978-0-12-814926-3.00009-7 [DOI] [Google Scholar]
- Ni K, Lan G, Chan C, Quigley B, Lu K, Aung T, … Lin W (2018). Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nature Communications, 9(1), 2351 10.1038/s41467-018-04703-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni K, Lan G, Veroneau SS, Duan X, Song Y, & Lin W (2018). Nanoscale metal-organic frameworks for mitochondria-targeted radiotherapy-radiodynamic therapy. Nature Communications, 9(1), 4321 10.1038/s41467-018-06655-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NNI Supplement to the President’s 2019 Budget [Budget Supplement]. (2018). Retrieved March 23, 2019, from U.S. National Nanotechnology Initiative website: https://www.nano.gov/sites/default/files/NNI-FY19-Budget-Supplement.pdf
- O’Kane PT, & Mrksich M (2017). An Assay Based on SAMDI Mass Spectrometry for Profiling Protein Interaction Domains. Journal of the American Chemical Society, 139(30), 10320–10327. 10.1021/jacs.7b03805 [DOI] [PubMed] [Google Scholar]
- Park S, Wong DJ, Ooi CC, Kurtz DM, Vermesh O, Aalipour A, … Gambhir SS (2016). Molecular profiling of single circulating tumor cells from lung cancer patients. Proceedings of the National Academy of Sciences, 113(52), E8379–E8386. 10.1073/pnas.1608461113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil R, Portilla-Arias J, Ding H, Konda B, Rekechenetskiy A, Inoue S, … Ljubimova JY (2012). Cellular delivery of doxorubicin via pH-controlled hydrazone linkage using multifunctional nano vehicle based on poly(β-l-malic acid). International Journal of Molecular Sciences, 13(9), 11681–11693. 10.3390/ijms130911681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Herlihy KP, Napier ME, & Desimone JM (2011). PRINT: a novel platform toward shape and size specific nanoparticle theranostics. Accounts of Chemical Research, 44(10), 990–998. 10.1021/ar2000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, … Blakey DC (2013). Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Research, 73(8), 2412–2417. 10.1158/0008-5472.CAN-12-4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigodich AE, Randeria PS, Briley WE, Kim NJ, Daniel WL, Giljohann DA, & Mirkin CA (2012). Multiplexed Nanoflares: mRNA Detection in Live Cells. Analytical Chemistry, 84(4), 2062–2066. 10.1021/ac202648w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak K, Farrell D, Panaro NJ, Grodzinski P, & Barker AD (2010). The NCI Alliance for Nanotechnology in Cancer: achievement and path forward. WIRE: Nanomedicine and Nanobiotechnology, 2(5), 450–460. 10.1002/wnan.98 [DOI] [PubMed] [Google Scholar]
- Qin L, Vermesh O, Shi Q, & Heath JR (2009). Self-powered microfluidic chips for multiplexed protein assays from whole blood. Lab on a Chip, 9(14), 2016–2020. 10.1039/B821247C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai P, Mallidi S, Zheng X, Rahmanzadeh R, Mir Y, Elrington S, … Hasan T (2010). Development and applications of photo-triggered theranostic agents. Advanced Drug Delivery Reviews, 62(11), 1094–1124. 10.1016/j.addr.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Cheung HW, von Maltzhan G, Agrawal A, Cowley GS, Weir BA, … Bhatia SN (2012). Targeted tumor-penetrating siRNA nanocomplexes for credentialing the ovarian cancer oncogene ID4. Science Translational Medicine, 4(147), 147ra112. 10.1126/scitranslmed.3003778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Published by NCI Alliance. (2017, August 8). Retrieved from National Cancer Institute website: https://www.cancer.gov/sites/nano/research/alliance-research
- Rizzi G, Lee J-R, Dahl C, Guldberg P, Dufva M, Wang SX, & Hansen MF (2017). Simultaneous Profiling of DNA Mutation and Methylation by Melting Analysis Using Magnetoresistive Biosensor Array. ACS Nano, 11(9), 8864–8870. 10.1021/acsnano.7b03053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzano G, Navarro G, Trivedi MS, De Rosa G, & Torchilin VP (2015). Multifunctional Polymeric Micelles Co-loaded with Anti-Survivin siRNA and Paclitaxel Overcome Drug Resistance in an Animal Model of Ovarian Cancer. Molecular Cancer Therapeutics, 14(4), 1075–1084. 10.1158/1535-7163.MCT-14-0556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton K, Tichauer K, Samkoe KS, Gunn J, Hoopes PJ, & Pogue BW (2013). Fluorescent affibody peptide penetration in glioma margin is superior to full antibody. PloS One, 8(4), e60390. 10.1371/journal.pone.0060390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MG, Goodwill PW, Neogy A, Yin M, Foster FS, Schaffer DV, & Conolly SM (2014). Biogenic gas nanostructures as ultrasonic molecular reporters. Nature Nanotechnology, 9(4), 311–316. 10.1038/nnano.2014.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Kotamraju VR, Mölder T, Tobi A, Teesalu T, & Ruoslahti E (2017). Tumor-Penetrating Nanosystem Strongly Suppresses Breast Tumor Growth. Nano Letters, 17(3), 1356–1364. 10.1021/acs.nanolett.6b03815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Rodriguez-Aguayo C, Xu R, Gonzalez-Villasana V, Mai J, Huang Y, … Lopez-Berestein G (2013). Enhancing chemotherapy response with sustained EphA2 silencing using multistage vector delivery. Clinical Cancer Research, 19(7), 1806–1815. 10.1158/1078-0432.CCR-12-2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Kantoff PW, Wooster R, & Farokhzad OC (2017). Cancer nanomedicine: progress, challenges and opportunities. Nature Reviews. Cancer, 17(1), 20–37. 10.1038/nrc.2016.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, & Jemal A (2019). Cancer statistics, 2019. CA: A Cancer Journal for Clinicians, 69(1), 7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- Skakuj K, Wang S, Qin L, Lee A, Zhang B, & Mirkin CA (2018). Conjugation Chemistry-Dependent T-Cell Activation with Spherical Nucleic Acids. Journal of the American Chemical Society, 140(4), 1227–1230. 10.1021/jacs.7b12579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Dave S, Nie S, True L, & Gao X (2006). Multicolor quantum dots for molecular diagnostics of cancer. Expert Review of Molecular Diagnostics, 6(2), 231–244. 10.1586/14737159.6.2.231 [DOI] [PubMed] [Google Scholar]
- Smith TL, Yuan Z, Cardó-Vila M, Sanchez Claros C, Adem A, Cui M-H, … Arap W (2016). AAVP displaying octreotide for ligand-directed therapeutic transgene delivery in neuroendocrine tumors of the pancreas. Proceedings of the National Academy of Sciences, 113(9), 2466–2471. 10.1073/pnas.1525709113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigliano RV, Shubitidze F, Kekalo K, Baker I, Giustini AJ, & Hoopes PJ (2013). Understanding mNP Hyperthermia for cancer treatment at the cellular scale. Proceedings of SPIE--the International Society for Optical Engineering, 8584, 85840E. 10.1117/12.2007518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, … Ruoslahti E (2009). Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell, 16(6), 510–520. 10.1016/j.ccr.2009.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taton TA, Mirkin CA, & Letsinger RL (2000). Scanometric DNA array detection with nanoparticle probes. Science (New York, N.Y.), 289(5485), 1757–1760. [DOI] [PubMed] [Google Scholar]
- Uckun FM, & Qazi S (2014). SYK as a New Therapeutic Target in B-Cell Precursor Acute Lymphoblastic Leukemia. Journal of Cancer Therapy, 5(1), 124–131. 10.4236/jct.2014.51015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Jianbin, Fan HC, Behr B, & Quake SR (2012). Genome-wide single-cell analysis of recombination activity and de novo mutation rates in human sperm. Cell, 150(2), 402–412. 10.1016/j.cell.2012.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Jun, Tham D, Wei W, Shin YS, Ma C, Ahmad H, … Heath JR (2012). Quantitating cell-cell interaction functions with applications to glioblastoma multiforme cancer cells. Nano Letters, 12(12), 6101–6106. 10.1021/nl302748q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Yanju, Lin W-Y, Liu K, Lin RJ, Selke M, Kolb HC, … Tseng H-R (2009). An integrated microfluidic device for large-scale in situ click chemistry screening. Lab on a Chip, 9(16), 2281–2285. 10.1039/b907430a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Yuhua, Xu Z, Guo S, Zhang L, Sharma A, Robertson GP, & Huang L (2013). Intravenous delivery of siRNA targeting CD47 effectively inhibits melanoma tumor growth and lung metastasis. Molecular Therapy, 21(10), 1919–1929. 10.1038/mt.2013.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmann JK, Bonomo L, Carla Testa A, Rinaldi P, Rindi G, Valluru KS, … Gambhir SS (2017). Ultrasound Molecular Imaging With BR55 in Patients With Breast and Ovarian Lesions: First-in-Human Results. Journal of Clinical Oncology, 35(19), 2133–2140. 10.1200/JCO.2016.70.8594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, … Wickline SA (2003). Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation, 108(18), 2270–2274. 10.1161/01.CIR.0000093185.16083.95 [DOI] [PubMed] [Google Scholar]
- Winter PM, Schmieder AH, Caruthers SD, Keene JL, Zhang H, Wickline SA, & Lanza GM (2008). Minute dosages of alpha(nu)beta3-targeted fumagillin nanoparticles impair Vx-2 tumor angiogenesis and development in rabbits. FASEB Journal, 22(8), 2758–2767. 10.1096/fj.07-103929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witney TH, Hoehne A, Reeves RE, Ilovich O, Namavari M, Shen B, … Gambhir SS (2015). A Systematic Comparison of 18F-C-SNAT to Established Radiotracer Imaging Agents for the Detection of Tumor Response to Treatment. Clinical Cancer Research, 21(17), 3896–3905. 10.1158/1078-0432.CCR-14-3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Siah KW, & Lo AW (2018). Estimation of clinical trial success rates and related parameters. Biostatistics. 10.1093/biostatistics/kxx069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Smith AM, Agrawal A, Ruan G, & Nie S (2006). Molecular profiling of single cancer cells and clinical tissue specimens with semiconductor quantum dots. International Journal of Nanomedicine, 1(4), 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Ramishetti S, Tseng Y-C, Guo S, Wang Y, & Huang L (2013). Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. Journal of Controlled Release, 172(1), 259–265. 10.1016/j.jconrel.2013.08.021 [DOI] [PubMed] [Google Scholar]
- Yang G, Qian X, Phan T, Sprenger F, Sultana S, Calderon-Colon X, … Zhou O (2011). Design and feasibility studies of a stationary digital breast tomosynthesis system. Nuclear Instruments & Methods in Physics Research. Section A, Accelerators, Spectrometers, Detectors and Associated Equipment, 648(Suppl 1), S220–S223. 10.1016/j.nima.2010.11.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Iyer AK, Singh A, Choy E, Hornicek FJ, Amiji MM, & Duan Z (2015). MDR1 siRNA loaded hyaluronic acid-based CD44 targeted nanoparticle systems circumvent paclitaxel resistance in ovarian cancer. Scientific Reports, 5, 8509 10.1038/srep08509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi K, Godin B, Oborn CJ, Alexander JF, Liu X, Fidler IJ, & Ferrari M (2013). Porous silicon nanocarriers for dual targeting tumor associated endothelial cells and macrophages in stroma of orthotopic human pancreatic cancers. Cancer Letters, 334(2), 319–327. 10.1016/j.canlet.2012.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong K-T, Hu R, Roy I, Ding H, Vathy LA, Bergey EJ, … Prasad PN (2009). Tumor targeting and imaging in live animals with functionalized semiconductor quantum rods. ACS Applied Materials & Interfaces, 1(3), 710–719. 10.1021/am8002318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CC, Blackley BW, Porter MD, & Granger MC (2016). Frequency-Domain Approach To Determine Magnetic Address-Sensor Separation Distance Using the Harmonic Ratio Method. Analytical Chemistry, 88(4), 2015–2020. 10.1021/acs.analchem.5b04271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue J, Pallares RM, Cole LE, Coughlin EE, Mirkin CA, Lee A, & Odom TW (2018). Smaller CpG-Conjugated Gold Nanoconstructs Achieve Higher Targeting Specificity of Immune Activation. ACS Applied Materials & Interfaces, 10(26), 21920–21926. 10.1021/acsami.8b06633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Xiong C, Huang M, Zhou M, Huang Q, Wen X, … Li C (2011). Peptide-conjugated polymeric micellar nanoparticles for Dual SPECT and optical imaging of EphB4 receptors in prostate cancer xenografts. Biomaterials, 32(25), 5872–5879. 10.1016/j.biomaterials.2011.04.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Bethune MT, Malkova N, Sutherland AM, Comin-Anduix B, Su Y, … Heath JR (2018). A kinetic investigation of interacting, stimulated T cells identifies conditions for rapid functional enhancement, minimal phenotype differentiation, and improved adoptive cell transfer tumor eradication. PloS One, 13(1), e0191634. 10.1371/journal.pone.0191634 [DOI] [PMC free article] [PubMed] [Google Scholar]


