Figure 10.
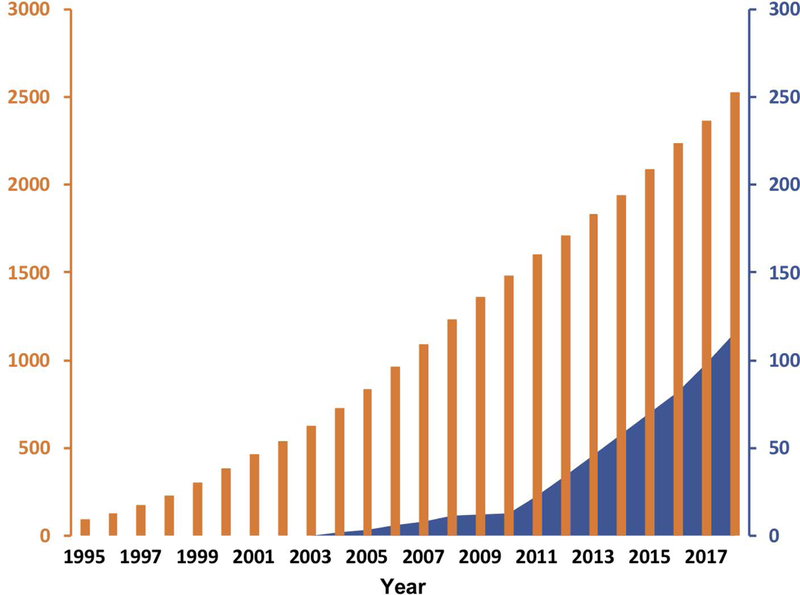
Cumulative cancer clinical trials conducted for liposomal versus non-liposomal nanoparticles over time. This graph displays clinical trials performed for liposomal delivery systems (solid black bars – left axis) since the approval of Doxil in 1995. In comparison, all non-liposomal nanoparticle cancer clinical trials are displayed as solid blue fill, corresponding right axis. The information was derived from clinicaltrials.gov based on the following keyword searches ‘Cancer and – liposomal, Caelyx, Myocet, Lipodox, Onivyde, Depocyt, Daunoxome, Marquibo, Vyxeos, or Mepact’ and for non-liposomal ‘Cancer and nanoparticle or nanotechnology’. The nab-paclitaxel trials that were found were excluded from non-liposomal trials count. The year on the horizontal axis corresponds to the initiation of the trial.
