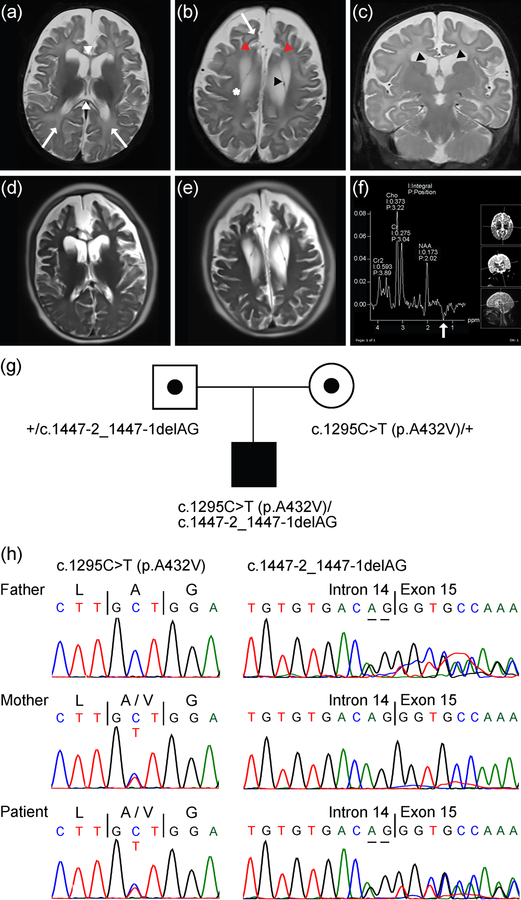
Figure 1.
MRI and MRS findings in male patient with compound heterozygous SLC25A12 pathogenic variants. (a-c) T2-weighted images at 7 months of age, with axial images shown in (a) and (b) and a coronal image in (c). Images reveal diffusely accentuated signal in the bilateral frontal and parietal deep white matter (a,b, arrows) that contrasts with myelinating periventricular and corticospinal tract white matter (b, *). The corpus callosum (a, white arrowheads) is abnormally thin for age, which is also reflective of white matter volume loss. Images also reveal diffuse panlobar sulcal prominence and white matter volume loss that results in contraction of the gyri, most conspicuously in the bilateral superior frontal gyri (b, red arrowheads). Thin membranes in the bilateral lateral ventricles that may outline intraventricular cysts (b,c, black arrowheads) can also be seen. (d,e) Axial HASTE images at 10 years 5 months of age suggest further contraction of the gyri and white matter, yet also reveal interval myelination on the T2-weighted images. (f) Results of MRS performed over the left basal ganglia at 7 months of age. The spectrum acquired at intermediate echo time (TE 135 ms) shows an inverted lactate peak at 1.3 ppm (white arrow), which may reflect sequela of seizures. (g) Pedigree showing heterozygous parents, each of which is a carrier of a distinct SLC25A12 variant, and the affected male patient with compound heterozygous variants in SLC25A12. Note, information regarding siblings of the patient is not shown to aid in protecting the anonymity and privacy of the family. (h) Sanger DNA sequencing chromatograms showing SLC25A12 variants in the father, mother, and patient, as indicated in (g). References for variant coordinates are based on GenBank transcript ID NM_003705 and Ensembl transcript ID ENST00000422440.