Fig. 2.
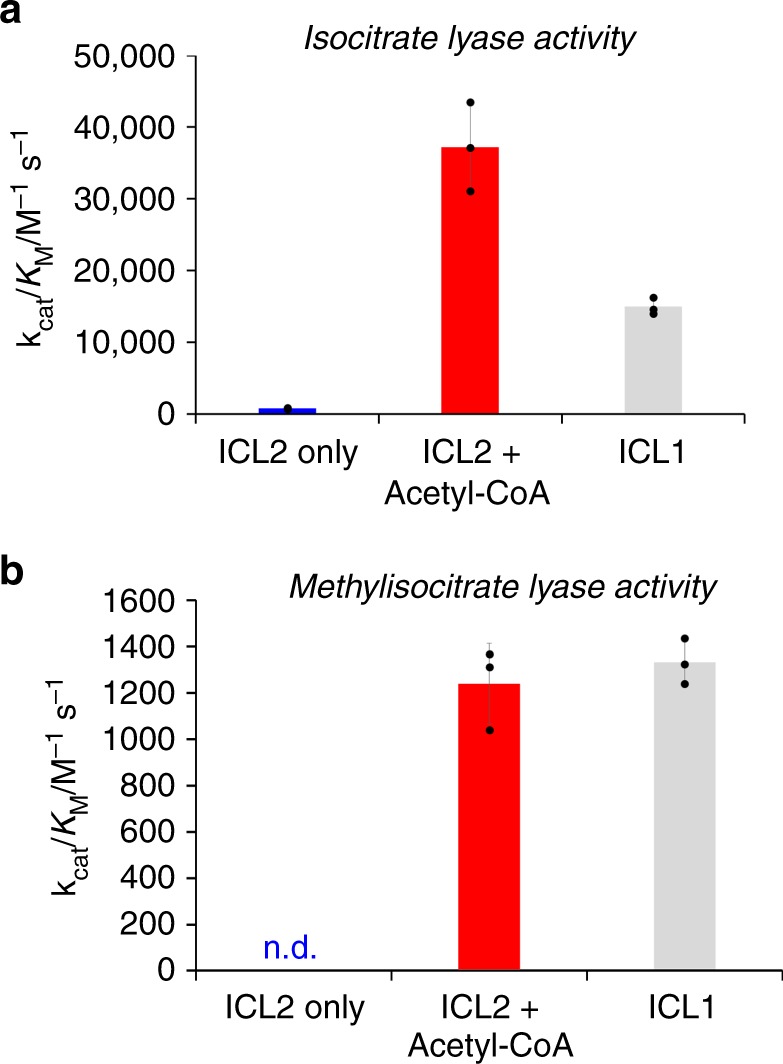
The catalytic activity of M. tuberculosis ICL2 is modulated by acetyl-CoA. a The catalytic efficiency of ICL2 is increased ~50-fold upon addition of acetyl-CoA with DL-isocitrate as a substrate. Reactions in the presence of acetyl-CoA were conducted with 0.2 μM ICL2, 100 μM–1 mM DL-isocitrate, 25 μM acetyl-CoA, 5 mM MgCl2 in 50 mM Tris-D11 pH 7.5 in 90% H2O and 10% D2O. Reactions in the absence of acetyl-CoA were conducted with 2 μM ICL2, 250 μM–2 mM DL-isocitrate, 5 mM MgCl2 in 50 mM Tris-D11 pH 7.5 in 90% H2O and 10% D2O. Reaction temperature was 27 °C. The uncorrected concentrations of the substrate DL-isocitrate were used. The error bars indicate standard deviations for three independent experiments. KM and kcat values of ICL1 were obtained from ref. 15. b While no detectable level of methylisocitrate turnover was observed with ICL2 only, acetyl-CoA also increased the catalytic efficiency of ICL2 when methylisocitrate was used as a substrate. The kcat/KM value in the presence of acetyl-CoA is comparable to the value obtained for ICL1. 2-Methylisocitrate was synthesised according to literature15. ICL2 reactions were conducted with 1 μM ICL2, 250 μM–2 mM 2-methylisocitrate, 25 μM acetyl-CoA, 5 mM MgCl2 in 50 mM Tris-D11 pH 7.5 in 90% H2O and 10% D2O. ICL1 reactions were conducted with 2 μM ICL1, 250 μM–2 mM 2-methylisocitrate, 5 mM MgCl2 in 50 mM Tris-D11 pH 7.5 in 90% H2O and 10% D2O. Reaction temperature was 27 °C. The error bars indicate standard deviations for three independent experiments. Source data are provided as a Source Data file
