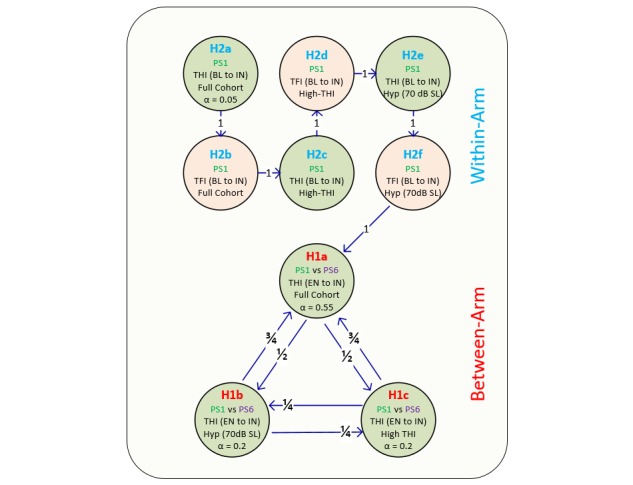
Hypothesis testing accounting for multiple comparisons. The primary endpoints for the TENT-A2 study correspond to several parallel and serial hypotheses depicted in the figure. A P value of .05 is initially distributed across four hypotheses (H1a, H1b, H1c, H2a), in which the portion of the P value attributed to each hypothesis is indicated by the alpha value. For example, alpha equals 0.55 for H1a, which corresponds to the null hypothesis being rejected if P<.0275 (.55x.05). The null hypothesis for H1a is that there is no between-arm difference in changes in mean Tinnitus Functional Index score from enrollment to interim (6-week timepoint) between parameter setting (PS) 1 and PS6 for the full cohort of participants. The null hypothesis for H1b and H1c is that there is no between-arm difference in changes in mean THI score from enrollment to interim between PS1 and PS6 for the hyperacusis subgroup and high tinnitus symptom severity subgroup, respectively. Both are rejected if P<.01 (.2x.05). The null hypothesis for H2a is that there is no within-arm change in THI from baseline (average of screening and enrolment scores) to interim for the full cohort of participants. H2a is rejected if P<.0025 (.05x.05). Note that the remaining hypotheses (H2b, H2c, H2d, H2e, H2f) can only be tested if the previous hypothesis in the series is rejected. For example, if H2a is rejected, then its portion of the P value (P=.0025) is transferred to H2b for testing. If H2b is rejected, then its portion of the P> value (P=.0025) is transferred to H2c, and so on. Similarly, the arrows shown for the between-arm comparisons indicate that if any of the other hypotheses (for H1a, H1b, or H1c) are successfully rejected, then their portion of the P value is distributed to its neighbors based on the proportion labeled on each arrow. The null hypothesis for the within-arm comparisons (H2a to H2f) is that there is no within-arm change in THI or Tinnitus Functional Index from baseline to interim for the full cohort of participants, hyperacusis subgroup, or high tinnitus symptom severity subgroup. Note that all within-arm comparisons will be based on a two-sided paired (dependent) t test, while all between-arm comparisons will be based on a linear regression with independent variables of treatment arm and THI score at enrollment. Further details on the statistical analysis plan are provided in the Statistical Methods section. BL: baseline, EN: enrollment, IN: interim, Hyp: hyperacusis subgroup (loudness discomfort level <70 dB sensation level at 500 Hz at screening), High-THI: high tinnitus symptom severity subgroup (THI >56 points at screening); TFI: Tinnitus Functional Index; THI: Tinnitus Handicap Inventory.
